Multiple roles of reactive oxygen species (ROS) and their consequences for health and disease are emerging throughout biological sciences.
This development has led researchers unfamiliar with the complexities of ROS and their reactions to employ commercial kits and probes to measure ROS and oxidative damage inappropriately, treating ROS (a generic abbreviation) as if it were a discrete molecular entity.
This can lead to misleading claims entering the literature and impeding progress, despite a well-established body of knowledge on how best to assess individual ROS, their reactions, role as signalling molecules and the oxidative damage that they can cause.
In this consensus statement we illuminate problems that can arise with many commonly used approaches for measurement of ROS and oxidative damage, and propose guidelines for best practice.
We hope that these strategies will be useful to those who find their research requiring assessment of ROS, oxidative damage and redox signalling in cells and in vivo.
Reactive oxygen species (ROS) (Box 1) are intimately involved in redox signalling but in some situations can also lead to oxidative damage.
Consequently, researchers from diverse fields often need to measure ROS, to assess oxidative events and to investigate their biological importance using antioxidants (Box 1) or inhibitors to modulate the phenomena observed.
There is a well-established field of biophysics/biochemistry/chemistry focusing on the identification of ROS, their chemical reactions and products of oxidative damage.
To address these points, this international group has set out guidelines on the nomenclature and measurement of ROS, oxidative reactions and oxidative damage.
Our focus is on the techniques used to measure ROS and oxidative damage.
Reactive oxygen species (ROS) is a collective term for species derived from O2 that are more reactive than O2 itself.
Hence all oxygen radicals are ROS, but not all ROS are radical species (the latter being defined as a species with one or more unpaired electrons).
Antioxidant is a term often used but difficult to define clearly.
what target of damage by ROS is measured.
One definition of an antioxidant is "any substance that delays, prevents or removes oxidative damage to a target molecule"1.
An alternative definition is "a substance that reacts with an oxidant to regulate its reactions with other targets, thus influencing redox-dependent biological signalling pathways and/or oxidative damage".
Oxidative damage: the biomolecular damage caused by the attack of ROS upon the constituents of living organisms.
Increased levels of oxidative damage can occur from increased ROS production but also from decreased repair or removal processes—for example, failure to clear oxidized proteins or repair oxidized DNA sufficiently rapidly: both can happen in certain diseases.
Biomarker: can be defined as any substance, structure or process that can be measured in the body or its products and influence or predict the incidence of outcome or disease54.
One problem that underlies the measurement of ROS and oxidative damage and the use of ‘antioxidants’ is the lack of precision in the use of these terms.
ROS is an abbreviation that covers a wide range of chemical species with different properties, reactivities and interactions (Box 1, Table 1).
For example, one important reactive species found in biology, the superoxide radical anion (O2•−), is formed by the one-electron reduction of oxygen (O2).
H2O2 is a substrate for haem peroxidases such as myeloperoxidase, generating further reactive species such as HOCl (Table 1).
A (far from complete) list of the physicochemical properties of the most common ROS encountered in biology is given in Table 1, which provides insights into what reactions might be plausible in vivo when these species are generated.
What should also be evident is that ‘reactive’ is highly context dependent, because the reactivity of different ROS varies over a wide scale, as do their lifespans, ability to diffuse and potential to generate further downstream reactive species.
The generalization ‘ROS’, although widely used (including in this paper!) does not give information about the actual chemical species causing the observed effect.
Recommendation 1: wherever possible, the actual chemical species involved in a biological process should be stated and consideration given to whether the observed effect is compatible with its reactivity, lifespan, products generated and fate in vivo.
These include enzymes and small molecules that react with individual ROS to decrease oxidative damage and/or modulate redox signalling1,2.
Often the effect of a putative antioxidant on a biological outcome is used to infer a role for a ROS, as if all antioxidants were equivalent.
For example, N-acetylcysteine (NAC) is a widely used ‘antioxidant’ but it has other (and sometimes more important18) modes of action.
It can also increase the cellular Cys pool and thereby enhance glutathione (GSH) levels, generate H2S, and directly cleave protein disulphides18.
Sometimes ‘•OH scavengers’ are used to infer a role for this ROS yet they can rarely, if ever, achieve a sufficiently high concentration to prevent the effectively instantaneous reaction of •OH with biomolecules1,7,13.
Other agents often used as ‘antioxidants’, such as TEMPO/TEMPOL, mito-TEMPO and porphyrin-based ‘SOD mimetics’, undergo complex redox reactions in vivo and are better described as ‘redox modulators’ rather than ‘antioxidants’ or ‘O2•− scavengers’1,20,21.
Recommendation 2: for an intervention to be attributed to an antioxidant activity, the particular chemical species targeted by the ‘antioxidant’ needs to be made explicit.
Wherever possible the activity of the antioxidant should be confirmed by measuring a decrease in oxidative damage.
A key procedure to attributing oxidative damage, or activation of a redox signalling pathway, to a particular ROS can be by selective generation of the ROS in a biological context.
Glucose oxidase can be used to generate H2O2 directly in vitro, while the regulated generation of H2O2 within cells can be achieved using genetically expressed d-amino acid oxidase, an enzyme that generates H2O2 as it oxidizes d-amino acids23.
NADPH oxidase (NOX) enzymes are important sources of O2•−and H2O2 for redox signalling, as well as oxidative damage9,24, and modulation of their activity is an important approach to understanding these processes.
Specific inhibitors24 or deletion or knockdown of NOX components should be used to identify their roles.
This can be done using electron paramagnetic (spin) resonance (EPR/ESR), various probe molecules or by measuring oxidative modifications (‘oxidative damage’, Box 1) caused by the ROS1.
Most ROS probes capture only a small percentage of any ROS formed.
Indeed, if the probe reacted with most of the ROS generated this would perturb the system and affect experimental results (for example, inhibition of oxidative damage or interference with redox signalling).
Oxidative damage can take many forms; the chemical processes by which it arises from a particular ROS and how it is assessed and quantified are complex.
Furthermore, the final level of any oxidative damage biomarker measured is the difference between its rate of production and its removal by repair, degradation, excretion or diffusion.
Recommendation 4: when oxidative damage levels to any biomolecule are presented, the chemical processes by which they arise and the methods used to quantify them should be made explicit.
Consideration of ROS, antioxidants and oxidative damage as monolithic concepts limits the precision and interpretation of experiments and glosses over the need to establish precise molecular mechanisms.
To put these precepts into practice requires measurement of specific ROS and/or oxidative damage products, as well as the effects of antioxidants.
The bottom line is that all methods used to assess ROS are susceptible to artefact, and appropriate controls are required to be certain of the species and amounts measured.
Commonly used cell culture conditions promote oxidative damage due to both limited antioxidants in the medium and high O2 concentrations relative to those in vivo26.
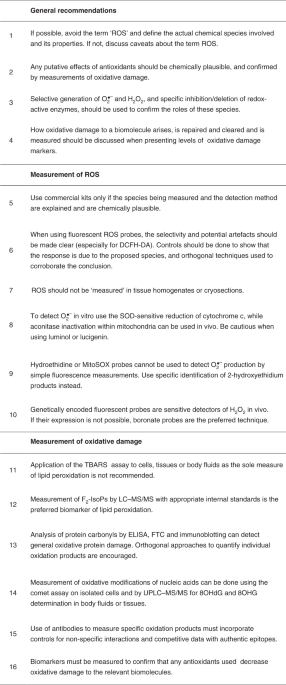
Recommendation 5: use commercial kits only if the actual species being measured and the method of detection are explained in the kit materials, are chemically plausible and the limitations are understood?
Small-molecule fluorescent probes are frequently used to assess ROS within cells.
In some cases, often involving kits, a lack of description of the chemical reactivity or structures of these probes makes it difficult to interpret results and therefore such probes should be avoided.
Even for probes of known structure there can be concerns.
Consider the widely used fluorescent probe 2',7'-dichlorodihydrofluorescein (DCFH), usually administered in its diacetate (DCFH-DA) form, which enters cells readily.
DCFH is not oxidized directly by H2O2 (which it is often claimed to detect), but only after H2O2 is converted to more reactive species by redox-active metals or by haem proteins such as cytochrome c or peroxidases.
This is not to say that DCFH, and other non-specific fluorescent probes such as dihydrorhodamine, should never be used, but their limitations (selectivity, problems of quantification, linearity of response and susceptibility to artefact) should be understood and results interpreted cautiously28.
While many small-molecule and protein fluorescent probes are more selective than DCF, it is always important to validate data by a number of simple controls: does the response change over time and with the amount of biological sample in a plausible manner.
Recommendation 6: when using fluorescent ROS probes (especially DCFH-DA), the chemistry involved, the selectivity for particular chemical species and potential artefacts should be made clear and discussed.
Wherever possible, controls to show that the response is due to the proposed species should be carried out and orthogonal techniques used to corroborate the conclusion.
There are valid methods available to assess ROS in vivo or in perfused organs, but in these situations the process is either monitored in vivo (for example, see Table 2 for the use of catalase compound I to measure H2O2) or the system is quenched to stabilize the probe for analysis ex vivo.
ROS should not be ‘measured’ in tissue homogenates or cryosections, unless the probe or sensor employed is able to irreversibly capture the reactive species when the cells/tissues/organs are under biologically relevant conditions.
The chemiluminescent ‘superoxide probes’ luminol and lucigenin are widely used to ‘detect O2•−’, but interpretation of such data is difficult because these probes generate radicals that produce O2•− themselves; they do not react with O2•− directly31,32.
Recommendation 8: the use of luminol and lucigenin to ‘detect O2•−’ should be discouraged, but they can be used as general indicators of increased ROS production.
Unfortunately, detection by fluorescence is misleading because these probes form both ethidium (E+), a non-specific oxidation product, and the O2•−-specific product 2-hydroxyethidium.
Because these two products have overlapping fluorescence spectra, it is hard to differentiate the contribution of non-specific oxidation and O2•−-dependent oxidation (if any) to the overall fluorescence33.
Another factor that should be considered is the extent of cellular uptake of HE/MitoSOX and the intracellular concentrations of these and their multiple products.
Furthermore, HE oxidation products intercalate into DNA, greatly enhancing their fluorescence and creating another artefact.
Mitochondrially accumulated O2•−probes, such as MitoSOX, are often used to ‘detect O2•−’ within mitochondria.
When using these probes, and others that have positive charges or generate positively charged species (including 2-hydroxyethidium and ethidium), it is important to remember that probe accumulation is dependent on plasma and mitochondrial membrane potentials and mitochondrial size, shape and mass35.
Recommendation 9: use only HE or MitoSOX probes to detect O2•−by simple fluorescence measurements when the product has been independently validated as 2-hydroxyethidium.
Fluorescence measurements with probes such as dihydroethidium and MitoSOX33 should be conducted using the lowest probe concentration possible, and must include controls for changes in plasma and mitochondrial membrane potentials and mitochondrial mass and morphology, such as normalization to a similar membrane-potential-responsive, but redox-insensitive, probe.
In simple systems, H2O2 can be measured by horseradish peroxidase (HRP)-oxidizing substrates, one frequently used being Amplex Red.
Since H2O2 can cross membranes directly or via aquaporins, this system can also be used to measure H2O2 release from cells.
However, please be aware that this release reflects the balance between H2O2 production, removal by intracellular enzymes and the rate of diffusion out of the cell.
Within cells, H2O2 detection by phenylboronate-based probes is more reliable38 although these may lack sufficient sensitivity because they react only slowly with H2O2, which can make it difficult to detect small or localized changes in H2O2 levels39.
However, recent studies suggest that borinic acids, which react more rapidly with H2O2, may be more sensitive detectors40.
The mechanism of oxidation of phenylboronates to phenols requires a two-electron oxidant, such as H2O2.
Because H2O2 is typically generated at higher concentrations than other ROS, boronate probes can be selective for H2O2 detection subject to proper controls39,40.
However, boronate probes react with ONOO−/ONOOH or HOCl much more rapidly than they do with H2O2 which can sometimes complicate measurements, and orthogonal approaches or the use of inhibitors can aid validation41.
For example, H2O2- and peroxynitrite-dependent signals can be distinguished using nitric oxide synthase (NOS) inhibitors and catalase38,39,41,42.
Genetically encoded fluorescent protein sensors have provided major advances in cellular H2O2 detection43,44,45,46.
These probes contain a dithiol switch that changes the overall fluorescence of the probe depending on its oxidation status.
High sensitivity and specificity for H2O2 have been achieved by coupling a redox-sensitive green fluorescent protein (GFP) mutant to a H2O2-sensitive thiol protein, such as oxyR (HyPer series), or to a peroxidase such as Orp1 or TSA2 (roGFP2-based probes).
While HyPer7- and roGFP2-based probes are pH stable, earlier versions of HyPer are not and require expression of a control probe (SypHer) to control for signal changes due to variation in pH43.
Imaging analysis by fluorescence microscopy is normally employed, but fluorescence plate readers can also be used.
The redox status measured represents a balance between the rate of oxidation and re-reduction of the probes by cellular reductants, including glutaredoxin/GSH and thioredoxin, permitting real-time, live-cell assessments of redox state.
Because excitation wavelengths of both reduced and oxidized probes are used, the probes are ratiometric and the output is not dependent on the level of protein probe expression.
By incorporation of appropriate targeting gene sequences, these probes can be directed to different cell compartments, including mitochondria, microtubules, endoplasmic reticulum, nucleus and cytoplasm43,44,45,46.
These probes have been expressed in transgenic animals to provide useful assessments of in vivo H2O2 generations46,47.
Plasmid transfection of viral vectors can be used with cultured cells, and targeted roGFP2 probes are available commercially (www.addgene.com).
In most experiments the H2O2 probes are expressed as free proteins that distribute within the cell.
Nevertheless, given uncertainties about intracellular H2O2 diffusion distances, it is still unclear what resolution is needed to understand subcellular H2O2 distribution.
Therefore, tethering H2O2 probes to sub-compartmental locations such as protein complexes or organelle contact sites is an important approach.
Recommendation 10: genetically encoded fluorescent probes (some of which are commercially available) are currently the most sensitive detectors of H2O2 and we recommend their use in cells and animals if expression is possible.
Boronate probes (some of which are also commercially available) are the preferred small-molecule probes, but controls to determine specificity for H2O2 are required and sensitivity is limited for physiological H2O2 levels.
Amplex Red with HRP can measure H2O2 release from cells if other reducing agents or peroxidase substrates are absent.
Products of this reaction include reactive species such as the carbonate radical anion (CO3•−) and the nitrating agent nitrogen dioxide (NO2•) (Table 1), both of which react with many of the general ‘ROS probes’.
Peroxynitrite oxidizes boronate-based probes nearly a million times more rapidly than H2O2 and, under the right conditions, these probes can be used to assess ONOO−/ONOOH production42,49.
HOCl, hypobromous acid (HOBr) and some of the chloramines and bromamines derived from them (Table 1) react with most of the general probes used to detect ROS, including DCFH and luminol.
However, many of these probes are also substrates of the peroxidases that generate HOCl or HOBr, confounding their use.
More specific fluorescent probes for reactive halogen species have been reported and some are commercially available52.
A genetically encoded probe for reactive halogen species has been developed, enabling dynamic monitoring of these species both in cell culture and in vivo53.
The presence of ROS can be inferred by their effects on protein, carbohydrates, nucleic acids and lipids to generate specific compounds which, so long as they cannot be formed by other mechanisms, can be used as ‘biomarkers’ of oxidative damage (Box 1)1,54,55.
However, do note that the measured levels of biomarkers represent a balance between the generation and removal of the biomarker (for example, by degradation, diffusion or excretion), plus any artefactual increased levels caused by oxidative damage during isolation or analysis.
Polyunsaturated fatty acids (PUFAs), are readily oxidized, and hence lipid peroxidation products are widely used to characterize oxidative damage56,57,58.
Thus, when measuring lipid peroxidation the focus might be placed on either (1) establishment of increased lipid peroxidation as an example of oxidative damage or (2) identification of individual oxidatively modified lipid molecules acting as signals by selective interaction with certain cellular targets.
LOO• can react further to yield highly oxidized secondary products, including epoxy-, oxo- or cyclic peroxides56,57,58.
Hence, there are multiple end products of lipid peroxidation that show vast chemical heterogeneity and variable stability and polarity, and thus measurement of only a single oxidation product in no way represents the whole process of lipid peroxidation.
Another fluorometric assay for lipid peroxidation employs cis-parinaric acid (PnA), a fatty acid with four conjugated double bonds.
In particular, 4-hydroxynonenal (HNE) formation has been widely used.
Antibodies against the protein adducts formed by HNE are widely available and frequently used in immunostaining of tissues, but it should be realized that different antibodies can detect different epitopes and so give different answers, depending on what amino acid residues the HNE binds to in proteins61,62,63,64.
One minor end product of lipid peroxidation is malondialdehyde (MDA)61, which can also be a useful biomarker if measured by MS techniques.
However, the widely used ‘MDA assays’ utilizing thiobarbituric acid-reactive substances (TBARS) are unspecific since TBA generates chromogens from many biomolecules other than MDA1,65.
Recommendation 11: application of the simple TBA test (TBARS), or kits based on its use, to cells, tissues or body fluids is not recommended as the only test used for evaluation of oxidative lipid damage because of the low specificity that can result in false-positive results.
The detection of lipid oxidation products has been revolutionized by the development of LC–MS for detailed analysis of oxidized lipid mixtures66.
Collection and storage of samples to avoid artefactual peroxidation is key to any lipid peroxidation study, and samples for later analysis should be immediately frozen in liquid nitrogen.
The internal standards used for quantification should be added to samples before solvent extraction.
Such LC–MS-based methods have the advantages of high sensitivity, small sample volume requirements and the ability to detect multiple end products of lipid peroxidation.
This makes LC–MS protocols the methods of choice for assessment of general lipid peroxidation and identification of individual products, including those with specific signalling functions.
Prominent among lipid oxidation products that have been quantified by MS-based approaches are the F2-isoprostanes (F2-IsoPs)69.
Recommendation 12: F2-IsoPs are a generally accepted biomarker of lipid peroxidation, but it should be realized that they are one of many end products and that the levels of various types can be affected by experimental conditions69.
Amino acid residues in proteins are sensitive to oxidative modification, some forms of which provide useful biomarkers74,75.
A common protein modification is the formation of ‘protein carbonyls’ due to oxidation of specific amino acid residues to carbonyl group-bearing products; carbonyls can also be formed by the reaction of aldehydes with nucleophilic sites on proteins or by glycation75,77.
Changes in protein carbonyls can be measured in tissue homogenates treated with fluorescein-5-thiosemicarbazide (FTC) to generate fluorophore-labelled proteins that can be separated by gel electrophoresis79.
Protein carbonyls, α-aminoadipic semialdehyde and glutamic semialdehyde have also been assayed individually by stable isotopic dilution analysis LC–MS/MS77.
This has been particularly useful in studies of oxidative damage to brain proteins in patients with dementia by ‘redox proteomics’81.
Peptide-level mapping after proteolytic cleavage allows detection of the nature of the modification, its location within the protein sequence and concomitant loss of the parent peptide, allowing relative quantification.
Amino acid analysis after complete digestion allows determination of types and absolute concentrations of particular species (determined by the use of isotope-labelled standards) together with the parent species, allowing determination of a ‘mass balance’75,76,77,82. Care must be taken in sample handling to prevent artefactual oxidation of Cys or Met, and also during protein hydrolysis, because some products of oxidative protein damage are labile. LC–MS analyses have many advantages including high specificity, high sensitivity, the capacity to detect many different modifications and parent species concurrently, as well as the capacity to detect products that are diagnostic of the ROS involved, such as chlorination from HOCl83 and nitrated species arising from the action of myeloperoxidase in the presence of NO2− and/or by reactions of ONOO−/ONOOH49,84).
Oxidation can be irreversible—for example, to a sulphinic or sulphonic acid, which can be useful biomarkers of oxidative protein damage4,85.
Recommendation 13: ELISA, FTC and immunoblotting are useful tools in the detection of protein carbonyls as a biomarker of general oxidative protein damage, although it must be realized that not all protein oxidation products contain carbonyls.
LC–MS approaches, using carefully prepared samples, are the best available techniques for assessment of protein oxidation due to the sensitivity, selectivity and quantitation available with these methods.
The use of orthogonal approaches, such as specific and validated antibodies (see below) against individual oxidation products, is also encouraged.
Oxidative modifications of DNA and RNA are often used as biomarkers of oxidative damage1,13,91.
One method used to assess ‘general’ oxidative damage to DNA in cells is the comet assay, which detects DNA strand breaks.
Such breaks can arise by several mechanisms, not necessarily via oxidative damage, but the use of repair enzymes that ‘nick’ DNA at the sites of oxidation increases the specificity for oxidative DNA damage.
Oxidative damage to DNA usually focuses on oxidation of guanine to 8-oxo-7,8-dihydro-2′-deoxyguanosine (8OHdG, or 8-oxodG).
The amount of 8OHdG (or any other product of oxidative DNA damage) measured in DNA is the balance between the rate of oxidation and that of repair.
Recommendation 14: when measuring oxidative modifications of nucleic acids from extracted cells or tissue samples, great care must be taken to avoid spurious oxidation in the preparative and analytical steps.
As discussed above, antibodies have been widely used to detect oxidation products (and also adducts) formed on proteins (for example, carbonyls and 3-nitro- and 3-chlorotyrosine), DNA (for example, 8-oxodG) and lipids (F2-Isoprostanes).
They have been used, for example, in ELISA, immunohistochemistry and immune precipitation formats, but often suffer from background reactivity, cross-reactivity and lack of specificity.
To address this, the epitope used to generate the antibody should be documented (for example, as for HNE)62,63,64 and controls to eliminate background should be included.
Recommendation 15: well-validated antibodies against specific products are useful detection tools when used with appropriate care and controls, including those for non-specific interactions.
EPR methods have been developed but are not yet widely used.
As noted earlier, genetically encoded redox biosensors have been used in animal studies.
With the development of improved sensitivity and detection modalities, positron emission tomography is now being used to image ROS in vivo98 but is still in its infancy.
In mitochondria of cells and tissues, changes in H2O2 can be assessed using the mitochondria-targeted boronate MitoB, which accumulates in these organelles and is converted by H2O2 into MitoP.
Because oxidative damage plays a central role in many human pathologies, there is considerable interest in developing therapeutic interventions to decrease this damage1,2,3.
A corollary is that in clinical trials we should be able to demonstrate how these interventions affect oxidative damage.
Unfortunately, in most cases the effect of the intervention on oxidative damage was not measured, making it uncertain whether the putative therapy was actually effective at decreasing oxidative damage: if it was not, lack of effect is predictable1,55.
To address this, it is essential to assess the impact of these interventions on levels of oxidative damage in the patients in clinical trials.
Currently, methods are limited to measuring end points of oxidative damage in either biopsies (for example, skin or muscle) or clinically accessible body fluids such as plasma, saliva, sputum or urine, and sometimes cerebrospinal fluid.
These biomarkers have included those for oxidation of nucleic acids such as 8OHG and 8OHdG100 and F2-isoprostanes as a biomarker of lipid peroxidation69,101.
To date, limited use has been made of biomarkers of protein oxidation in clinical trials.
However, there is evidence for strong associations of alterations in protein thiol/disulphide ratios, and increased protein carbonyls and other modifications with pathologies75,76,77.
Ideally a panel of biomarkers should be used54,55 since end products of oxidative damage to lipids, proteins and nucleic acids do not necessarily correlate with each other, nor would we expect them to since they are different molecular targets of different ROS.
Recommendation 16: if intervening with antioxidants, first use biomarkers in preliminary dose-ranging studies to determine whether the intervention does indeed decrease oxidative damage to the relevant biomolecules.
The goal of this consensus statement is to generate a useful resource for researchers from diverse fields who find themselves needing to measure ROS and to assess oxidative events to investigate their biological importance.
Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology.
Targeting oxidative stress in disease: promise and limitations of antioxidant therapyM
Google Scholar .
Google Scholar .
The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells.
Google Scholar .
Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses.
Google Scholar S
Google Scholar .
Google Scholar .
Google Scholar .
Unraveling the biological roles of reactive oxygen speciesA
Google Scholar .
Google Scholar .
Google Scholar .
Hydroxyl radical is a significant player in oxidative DNA damage in vivo.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Methionine in proteins: it’s not just for protein initiation anymoreC
Google Scholar .
The mechanism of action of N-acetylcysteine (NAC): the emerging role of H2S and sulfane sulfur species.
Google Scholar W
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Reactive oxygen species: not omnipresent but important in many locations.
Google Scholar .
Detection of superoxide and H2O2 produced by NADPH oxidasesR
Google Scholar .
Google Scholar .
Google Scholar .
Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides.
Google Scholar .
In vivo imaging of hydrogen peroxide with HyPer probes.
Google Scholar .
Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Biochemistry of peroxynitrite and protein tyrosine nitration.
Google Scholar .
Google Scholar .
Google Scholar .
Recent development of synthetic probes for detection of hypochlorous acid/hypochlorite.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Lipid Peroxidation in Biomembranes (CRC Press, 1988).
Lipid peroxidation: physiological levels and dual biological effects.
Google Scholar .
Free radical lipid peroxidation: mechanisms and analysis.
Google Scholar .
Mass spectrometric analysis demonstrates that BODIPY 581/591 C11 overestimates and inhibits oxidative lipid damage.
Google Scholar .
Parinaric acid as a sensitive fluorescent probe for the determination of lipid peroxidation.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Specificity of the ferrous oxidation of xylenol orange assay: analysis of autoxidation products of cholesteryl arachidonate.
Google Scholar .
Google Scholar H
Google Scholar .
Google Scholar .
Google Scholar .
Measurement of urinary F2-isoprostanes as markers of in vivo lipid peroxidation: a comparison of enzyme immunoassays with gas chromatography-mass spectrometry in domestic animal species.
Google Scholar .
Google Scholar ?
Google Scholar .
Protein damage and degradation by oxygen radicals: I general aspects.
Google Scholar .
Detection, identification, and quantification of oxidative protein modifications.
Google Scholar .
Quantification of protein oxidation by oxidants.
Google Scholar .
Reading patterns of proteome damage by glycation, oxidation and nitration: quantitation by stable isotopic dilution analysis LC-MS/MS.
Google Scholar ?
Protein carbonyl measurement by enzyme-linked immunosorbent assay.
Google Scholar .
Detection of protein carbonyls in aging liver tissue: a fluorescence-based proteomic approach.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Analysis of protein chlorination by mass spectrometry.
Google Scholar .
Google Scholar .
Introduction to approaches and tools for the evaluation of protein cysteine oxidation.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
A quantitative tissue-specific landscape of protein redox regulation during aging.
Google Scholar .
Google Scholar J
Google Scholar .
Google Scholar .
& ESCODD (European Standards Committee on Oxidative DNA Damage).Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study.
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar .
Google Scholar
In vivo reactive oxygen species detection with a novel positron emission tomography tracer, 18F-DHMT, allows for early detection of anthracycline-induced cardiotoxicity in rodents
Google Scholar
Google Scholar
Google Scholar
Google Scholar
Google Scholar
Google Scholar
Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo