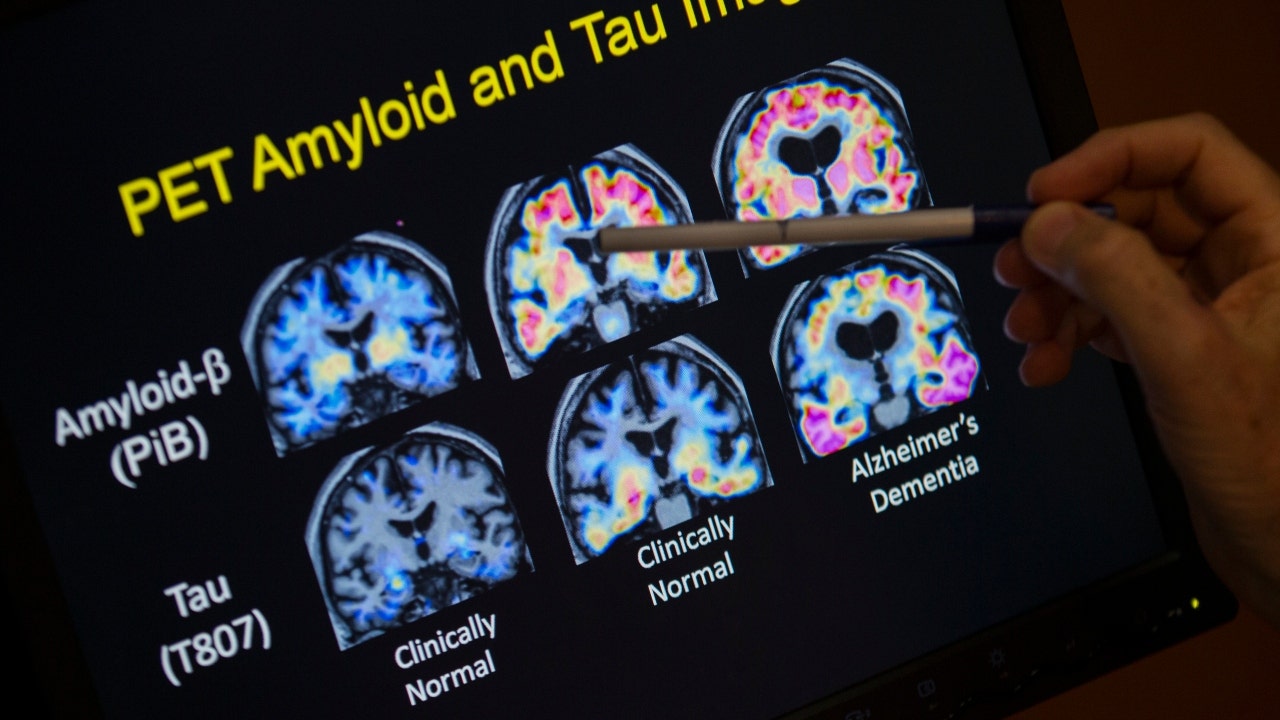
A score of 0.5 to 6 is consistent with early Alzheimer’s disease, according to the researchers. .
Officials at the Alzheimer’s Discovery Drug Foundation (ADDF) said in a released statement that lecanemab, which is up for FDA approval in early 2023, represents a positive step toward treatment of the disease and "welcomed news for the millions of patients and families living with Alzheimer’s."The Alzheimer’s Association said it was encouraged by the global clinical trial of lecanemab. "Unique drug combinations matched to each patient’s underlying pathologies is the answer and our best hope to give patients long-lasting relief from this insidious and progressive disease," Fillit said in the released statement. The Alzheimer’s Association also released a statement regarding the phase three-trial results.One expert who was not involved in the study told Fox News Digital she was excited to see the statistically significant difference between the lecanemab and placebo groups in the study — but cautioned that more research on the Alzheimer's drug is needed.
It said the study "confirms this treatment can meaningfully change the course of the disease for people in the earliest stages of Alzheimer’s diseaseThe Alzheimer’s Association calls for the Food and Drug Administration’s accelerated approval of lecanemab."
Gieniusz, who was not involved in the study, said she was excited to see the statistically significant difference between the lecanemab and placebo groups in the study — but cautioned that more research on the drug is needed.
"The FDA is expected to decide whether to grant accelerated approval to lecanemab by January 6, 2023," said the Alzheimer's Association.
"The FDA is expected to decide whether to grant accelerated approval to lecanemab by January 6, 2023," the association said