Here we show that, following CSDS, a subset of male and female mice, termed susceptible (SUS), avoid social interaction with non-aggressive, same-sex juvenile C57BL/6J mice and do not develop context-dependent social reward following encounters with them.
Non-social stressors have no effect on social reward in either sex.
Next, using whole-brain Fos mapping, in vivo Ca2+ imaging and whole-cell recordings, we identified a population of stress/threat-responsive lateral septum neurotensin (NTLS) neurons that are activated by juvenile social interactions only in SUS mice, but not in resilient or unstressed control mice.
Optogenetic or chemogenetic manipulation of NTLS neurons and their downstream connections modulates social interaction and social reward.
Together, these data suggest that previously rewarding social targets are possibly perceived as social threats in SUS mice, resulting from hyperactive NTLS neurons that occlude social reward processing.
Social avoidance manifests across a host of psychiatric illnesses, with causes ranging from disinterest in social contact6 to negative emotional states evoked by social encounters7.While the causes of social avoidance are diverse8, past social trauma can result in severe social avoidance thought to reflect reduced social reward2,9.
Despite a deep clinical understanding of social trauma and its resultant effects on social behaviour, we know very little about the underlying neural circuitry involved.
CSDS reduces exploratory behaviours and preference for natural rewards like sucrose, and results in severe social avoidance interpreted as social anhedonia5,10.
However, past CSDS studies assessed social interaction with an adult CD-1 mouse, similar to those used as aggressors to induce the social trauma.
Social avoidance under these circumstances probably reflects fear or submissive behaviour rather than impaired social reward.
To better understand whether social reward deficits are induced by CSDS, we assessed social behaviour by testing social interaction and social conditioned place preference (sCPP) with a non-threatening, same-sex juvenile C57BL/6J mouse that, under control conditions, is rewarding.CSDS—but not non-social chronic stressors like chronic variable stress (CVS)—blocks social reward in a subset of mice termed susceptible (SUS).
We next employed a circuit-based approach to better understand the mechanisms by which previous traumatic social experience with an adult male aggressor affects subsequent social reward processing.
Following CSDS, SUS mice exhibit heightened activity within lateral septum neurotensin (NTLS) neural circuitry, which occludes social reward and promotes sustained social avoidance behaviour even when presented with a non-threatening social situation.
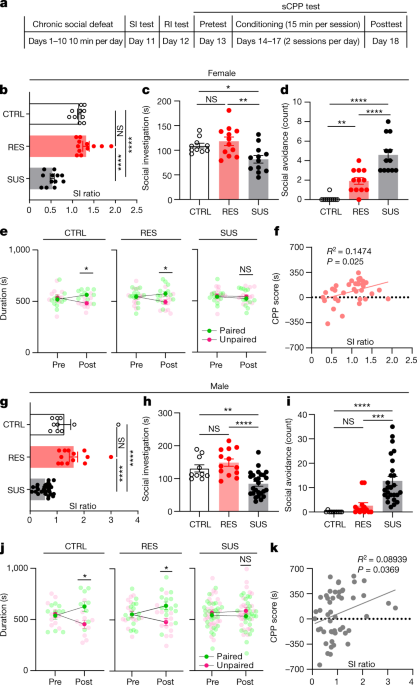
To investigate how CSDS affects social interaction and social reward, 7–8-week-old wild-type (WT) male and female mice underwent standard CSDS followed by social interaction testing with a CD-1 or ERα-Cre F1 mouse10,11.As previously described, mice were classified as either resilient (RES) or SUS based on their social interaction behaviour (that is, social interaction (SI) ratio) (Fig. 1a,b,g and Extended Data Fig. 1a,b,d,e).
This was followed by a resident intruder (RI) test and sCPP with 4–6-week-old, same-sex juvenile C57BL/6J mice.
During the RI test, control (CTRL) and RES mice exhibited similar social behaviours towards the juvenile, including the amount of active interaction (that is, approach, close following and sniffing).
Mice in these groups rarely withdrew when the juvenile approached and investigated, which we define as passive social investigation.
Conversely, SUS mice exhibited much less active social investigation, longer latency to the first social bout and significantly more social avoidance during passive social investigation with a juvenile (Fig. 1c,d,h,i and Extended Data Fig. 1c,f).
Social investigation time, social avoidance and latency to investigate correlated with SI ratio during testing with a CD-1 (Extended Data Fig. 1g–l).
These results show that SUS mice exhibit avoidance not only toward aggressive adult CD-1 male mice, but also toward non-threatening, same-sex C57BL/6J juvenile mice.
We next used the sCPP test to assess social preference; CTRL and RES, but not SUS, mice formed social preference to the intruder-paired context (Fig. 1e,j), suggesting that juvenile interaction is not rewarding to SUS mice.
sCPP score correlated with SI ratio (Fig. 1f,k) as well as social investigation time, social avoidance counts and latency to the first social bout during the RI test (Extended Data Fig. 1m–r).
The female oestrous cycle was not associated with any differences in sCPP formation (Extended Data Fig. 1s).
Interestingly, we found that female mice formed a significant sCPP only when the juvenile mice were confined to a wire-mesh cup during conditioning (Extended Data Fig. 1t), so we used this design for all studies in females.
All behavioural parameters were normally distributed except for social avoidance (Extended Data Fig. 1u).
Given that sCPP is dependent on intact learning and memory processes, we performed novel-object recognition and novel-location tests and found no evidence of learning and memory deficits in SUS or RES mice compared with CTRL mice (Extended Data Fig. 2a–c).
To test whether the order in which behavioural tests were performed affected aspects of social behaviour, we reversed the order of testing (sCPP–RI–SI) in WT mice following CSDS and found similar effects (Extended Data Fig. 2d,e).
Next, we grouped mice first by sCPP scores (social preference) and found a similar positive correlation with social investigation in the RI test along with a trend for SI ratio (Extended Data Fig. 2f,g), which again suggests that these different social behaviours largely correlate with one another.
Together, these data support the idea that CSDS-induced social avoidance results from disruptions in social reward processing, which led us to consider that SUS mice may in fact perceive juvenile social targets as threatening or stressful.
a, Experimental timeline for social behaviour tests following chronic social defeat.c,h, Social investigation time from RI test of females (one-way ANOVA, F (2, 31) = 6.755, P = 0.0037) (c); and of males (F (2, 46) = 14.82, P < 0.0001) (h).
d,i, Social avoidance of females (F (2, 31) = 33.13, P < 0.0001) (d) and males (F (2, 46) = 15.37, P < 0.0001) (i).
To investigate the circuit mechanisms mediating social reward deficits in SUS mice, we conducted a cleared whole-brain Fos mapping procedure using the iDISCO+ method12 to examine differentially modulated brain regions following CSDS when mice were exposed to juvenile intruders (Fig. 2a and Extended Data Fig. 3a–h).Cleared brains (Fig. 2b) were imaged on a lightsheet microscope (Fig. 2c), followed by registration and annotation (Extended Data Fig. 3i) using ClearMap12.
To first screen potentially relevant brain regions, Fos+ cells were compared among CTRL, SUS and RES mice to identify differentially regulated brain regions in both sexes (Fig. 2d, Extended Data Tables 1–5 and Supplementary Table 1).
Interestingly, we found dramatic sex-based differences in Fos activity when comparing RES and SUS mice, despite both sexes exhibiting similar social deficits.
Notably, the lateral septum (LS) was one of the most highly activated brain regions in both SUS males and females compared with RES mice, so we selected it for further investigation.
To confirm that Fos activation in SUS mice was due to the presence of a social target, we performed an additional RI test following CSDS with a novel object versus a juvenile intruder.
Under these conditions, we found that Fos activity was significantly higher in SUS mice following juvenile interaction compared with novel-object interaction.
Although we observed a slight increase in Fos activity following both novel-object and juvenile interaction in RES mice, there were no significant differences in time spent between them (Extended Data Fig. 3j,k).
g,h, Multiplex ISH (g) showing Fos expression (h) in NT neurons in females (one-way ANOVA, F (2, 6) = 7.887, P = 0.0209, n = 3 mice per group, three slices per mouse) and males (F (2, 10) = 13.13, P = 0.0016, n = 3 (CTRL), 4 (RES), 6 (SUS), three slices per mouse); scale bars, 50 μm.
j, eYFP+ NTLS neurons patched in whole-cell configuration.
NTLS neurons from SUS mice (n = 55 neurons) compared with RES mice (two-way ANOVA, P < 0.0001, n = 19).
Interestingly, in SUS mice following juvenile RI we found that most Fos-expressing neurons were located specifically in the lateral-ventral portion of the LS.
Several recent studies have examined the role of these molecularly defined cell types in regulation of behaviour, including Sst+ neurons in fear conditioning21, vGAT+ and Nt+ neurons in stress-suppressed feeding22,23, Crhr2+ neurons in anxiety-like behaviour24, as well as Oxtr+ and Drd3+ neurons in social fear25 and social dysfunction26.
To determine which cell type was activated by juvenile social interaction in SUS mice, we performed multiplex ISH on brain slices from CSDS mice following juvenile RI (Fig. 2f).
We found over 94% colocalization between Nt and Fos in SUS mice, with very limited expression of Fos in Nt– cells (Fig. 2g,h and Extended Data Fig. 4a).
Around 100% of all Nt+ cells were GABAergic (Extended Data Fig. 4b,c).
Interestingly, Nt and Crhr2 messenger RNA were largely colocalized in the anterior part but not in the posterior part of the LS, where we found significant increases in Fos levels following juvenile RI in SUS mice (Extended Data Fig. 4d,e).
Nt+ neurons had an overlap of about 5% with Drd3+ and of about 20% with Oxtr+ neurons (Extended Data Fig. 4f–i).
Interestingly, we also found an increase in Sst+ Fos+ neurons in SUS mice following juvenile social interaction relative to CTRL mice, but not between RES and SUS mice (Extended Data Fig. 4j,k).
Last, we found no differences in Nt– Fos+ neurons between CTRL, RES and SUS mice (Extended Data Fig. 4l).
Together these data highlight a potentially strong involvement of NTLS neurons in social reward deficits in SUS mice.
To confirm that NTLS neurons were indeed hyperactivated in SUS mice following interaction with a juvenile, we used a whole-cell slice electrophysiological approach to record NTLS neurons in defeated male mice following a juvenile RI test (Fig. 2i,j).We found that NTLS neurons from SUS mice showed increased excitability (Fig. 2k,n), as well as decreased resting membrane potential (Fig. 2l and Extended Data Fig. 5a), when compared with RES mice.
These changes in intrinsic properties of NTLS neurons suggest that CSDS induces lasting adaptations in these cells, which mediate social dysfunction.
Interestingly, we found no differences in other properties of these cells (action potential threshold, amplitude, half-width or fast hyperpolarization; Extended Data Fig. 5a), confirming that CSDS specifically increases the excitability of these cells in SUS mice.
To further investigate NTLS neuron activity in vivo during social encounters with juveniles, we injected Cre-dependent adeno-associated virus (AAV)-DIO-GCaMP6s into the LS of Nt-Cre transgenic mice to label NTLS neurons (Fig. 3a).We then measured fluorescent Ca2+ activity by fibre photometry (FP) in CTRL, RES and SUS mice during juvenile RI (Fig. 3b and Extended Data Fig. 5b).
We found no increase in NTLS neuron activity in CTRL (Fig. 3c,d) and RES (Fig. 3e,f) mice in response to juvenile approach, but SUS mice exhibited significantly higher activity (Fig. 3g,h).
Surprisingly, the magnitude (approximately 5–10% change in fluorescence (ΔF/F)) of increase in NTLS neuron activity during juvenile approach was similar to that observed when unstressed CTRL mice encountered an aversive experience, such as coming under attack by an aggressive CD-1 mouse (Fig. 3i,j) or experiencing a painful investigator-administered tail pinch (Fig. 3k,l).
Moreover, NTLS neuron activity showed no change following palatable food consumption (Extended Data Fig. 5c).
These findings are consistent with the idea that NTLS neurons respond to aversive, but not to rewarding, stimuli.
We further tested NTLS neuron activity during sCPP conditioning and found that NTLS neurons in SUS mice showed higher activity during the juvenile-paired conditioning session, with no changes observed in CTRL or RES mice (Extended Data Fig. 5d).
On the basis of these data, we suggest that, following CSDS, SUS mice may overgeneralize social threat cues and perceive juveniles as social threats, similar to that observed when being attacked by a highly aggressive CD-1 mouse.
c–h, Left, representative Ca2+ trace of NTLS neurons during resident intruder test (pink strips indicate passive social investigation); middle, peri-event plot of NTLS neuron activity 2 s before and after intruder approach; right, statistics for neuron activity 2 s before and 2 s after social events in CTRL females (paired two-tailed t-test, t6 = 3.379, P = 0.0149, n = 7) (c) and males (t6 = 0.5081, P = 0.6295, n = 7) (d); in RES females (t4 = 0.6528, P = 0.5495, n = 5) (e) and males (t4 = 0.2939, P = 0.7834, n = 5) (f); and in SUS females (t4 = 3.772, P = 0.0196, n = 5) (g) and males (t4 = 4.844, P = 0.0084, n = 5) (h).
i–l, Left, representative Ca2+ trace of NTLS neurons in CTRL mice during social defeat and tail pinches; middle, peri-event plot of NTLS neuron activity 2 s before and 2 s after attack/tail pinch; right, statistics of neuron activity 2 s before and 2 s after event in female defeat (t7 = 6.852, P = 0.0002, n = 8) (i), male defeat (t6 = 6.973, P = 0.0010, n = 7) (j), female tail pinch (t4 = 3.988, P = 0.0163, n = 5) (k) and male tail pinch (t5 = 6.137, P = 0.0017, n = 6) (l).
To assess whether NTLS neurons regulate social behaviours following CSDS, we utilized viral vectors expressing designer receptors exclusively activated by designer drugs (DREADDs) to bidirectionally manipulate the activity of NTLS neurons during SI with a CD-1 mouse, and also during juvenile RI and sCPP.About 3–4 weeks before CSDS, we injected AAV-DIO-hM3Dq, AAV-DIO-hM4Di or AAV-DIO-mCherry viruses into the LS of 4-week-old Nt-Cre mice (Fig. 4a and Extended Data Fig. 6a).
We found bidirectional effects of NTLS neuron modulation on SI in both females and males, with increased activity reducing SI in RES mice and decreased activity enhancing SI in SUS mice (Fig. 4b,i).
Inhibition of NTLS neurons increased social investigation time and normalized avoidance behaviour in both sexes (Fig. 4c,d,j,k).
For sCPP, we treated hM4Di-injected SUS mice and hM3Dq-injected RES mice with vehicle or CNO during social conditioning sessions.
We found that, by inhibiting NTLS neurons in SUS mice, we could normalize preference for the social conditioned compartment to CTRL or RES levels in both sexes (Fig. 4e,f,l,m).
Conversely, by activating NTLS neurons in RES mice, we were able to reduce social investigation and social preference compared with their vehicle-treated controls in both sexes (Fig. 4g,h,n,o).
Thus, we find that activation of NTLS neurons resulting from social trauma is both necessary and sufficient to elicit social behaviour deficits.
Interestingly, activation of NTLS neurons in stress-naïve mice affected neither SI ratio nor sCPP (Extended Data Fig. 6b–d), which suggests that a history of social trauma is critical.
In line with this, we find no effect of non-social stressors, such as CVS, on social reward (Extended Data Fig. 6e,f), despite the fact that both CSDS and CVS similarly reduce preferences for natural rewards like sucrose27.
Consistent with this, a recent study showed that ventral CA3 neurons projecting to the LS play a role in acute social stress-induced avoidance28, but not in unstressed mice29.
To test whether NTLS neurons can more generally regulate reward or aversion behaviour, we utilized a real-time place preference assay (RTPP) in stress-naïve mice and found no effect of optogenetic stimulation of NTLS neurons on preference (Extended Data Fig. 6g,h).
Taken together, these data support the idea that NTLS circuits modulate social behaviours in a context-dependent fashion.
b–d,i–k, SI ratio, social investigation and social avoidance following chemogenetic activation (RES mice) or inhibition (SUS mice) of NTLS neurons during social test following CSDS (two-way repeated-measures ANOVA, female: F (2, 53) = 9.785, P = 0.0002, n = 18 (hM3Dq), 20 (hM4Di), 16 (mCherry) (b), F (2, 25) = 5.807, P = 0.0085 (c), F (2, 25) = 5.906, P = 0.0079, n = 9 (hM3Dq), 10 (hM4Di), 8 (mCherry) (d); male: F (2, 64) = 12.96, P < 0.0001, n = 30 (hM3Dq), 20 (hM4Di), 17 (mCherry) (i), F (2, 20) = 19.46, P < 0.0001 (j), F (2, 20) = 10.12, P = 0.0009, n = 8 (hM3Dq), 8 (hM4Di), 7 (mCherry) (k)).
e–h,l–o, Social preference rescued by inhibition of NTLS neurons in female SUS mice (two-way repeated-measures ANOVA, CNO (e), F (1, 14) = 7.272, P = 0.0174, n = 8; vehicle (f), F (1, 14) = 0.3070, P = 0.5883, n = 8); and in male SUS mice (CNO (l), F (1, 14) = 4.710, P = 0.0477, n = 8; vehicle (m), F (1, 18) = 1.627, P = 0.2183, n = 10).
Activation of NTLS populations in RES females (two-way repeated-measures ANOVA, CNO (g), F (1, 18) = 0.1653, P = 0.6891, n = 10; vehicle (h), F (1, 18) = 8.490, P = 0.0093, n = 10); and in RES males (CNO (n), F (1, 14) = 0.2221, P = 0.6447, n = 8; vehicle (o), F (1, 16) = 9.283, P = 0.0077, n = 9) blocked social CPP formation.
To determine whether NTLS neurons encode context-specific information related to non-social stressors, we exposed WT mice to chronic restraint stress (CRS) and then performed an interaction test with a new restraint tube.We found that mice exposed to CRS had a longer latency to approach the tube and reduced time spent investigating the tube (Extended Data Fig. 7a,b).
We then silenced NTLS neurons with an inhibitory DREADD and found that this partially rescued tube avoidance (Extended Data Fig. 7c,d).
In a separate group, we paired WT mice with juvenile bedding/odour during CRS (CRSO) and found no differences in the juvenile RI test, suggesting that mice do not generalize avoidance to a juvenile social target based on exposure to these olfactory cues (Extended Data Fig. 7e,f).
Overall, these data suggest that NTLS neurons are involved in more general computations that use past information from stressful or threatening situations to guide future behaviours towards cues associated with the same or similarly threatening/stressful situations.
Last, we found a role for NTLS neurons in mediating anxiety-related behaviours, such as the elevated plus maze (EPM), marble burying test and open field test (OFT) (Extended Data Fig. 7g–j).
To determine the output patterns of NTLS neurons, we applied multiple viral-mediated anterograde tracing tools.
We then used HSV-1 (H129ΔTK-TT) for anterograde trans-synaptic tracing33 to verify which regions form monosynaptic connections with NTLS neurons (Fig. 5b and Extended Data Fig. 8a).
Interestingly, many of the downstream regions identified, such as the medial-lateral preoptic area (LPO/MPO), nucleus accumbens (NAc), anterior hypothalamic nucleus (AHN), lateral hypothalamus (LH), periaqueductal grey (PAG), medial amygdala (MEA) and supramammillary nucleus (SuM), are all involved in various aspects of social behaviour34 or conditioned reward35.
Among these regions, the NAc is involved in social reward36,37 and stress susceptibility35,38, and the PAG in social aggression39, as well as in defensive and escape behaviours40,41.
Although the AHN plays a role in defensive behaviour42 and parental behaviour43, its role in social reward remains unknown.
We wanted first to determine whether the same or different NTLS neurons project to each of these sites.
We injected a Cre-dependent retrograde AAV (rgAAV-DIO) expressing tdTomato into the AHN, NAc or PAG of Nt-Cre mice (Extended Data Fig. 8b).
In the same mice, rgAAV-DIO-eYFP was injected into the alternate regions and we visualized overlap between tdTomato and eYFP in NTLS neurons.
We also injected cholera toxin subunit B (CTB) into the NAc (CTB488), AHN (CTB555) and PAG (CTB647) (Extended Data Fig. 8d) and found similar results: AHN/NAc-, AHN/PAG- and NAc/PAG-projecting LS neurons showed little overlap (Extended Data Fig. 8c,e), further confirming that LS neurons projecting to these regions represent mostly separate subpopulations.
To investigate the function of these NTLS circuits, we injected AAV-DIO-ChR2(H134R) into the LS of 5-week-old Nt-Cre mice and implanted ferrules in the NAc, AHN or PAG.
Three weeks later, mice underwent a subthreshold CSDS (stCSDS) and social behaviour was tested during a 2-day, 5 min juvenile RI test in which laser on/off order was counterbalanced (Fig. 5c).
Activation of NTLS→AHN or NTLS→NAc circuits decreased active social investigation time without affecting social avoidance behaviour during passive social bouts initiated by the juvenile (Fig. 5d–i).
However, activation of NTLS→PAG circuit had no effect on either social investigation time or social avoidance (Fig. 5j–l).
To further validate whether manipulation of NTLS→AHN or NTLS→NAc circuits can bidirectionally modulate social interaction, we injected AAV-DIO-eNpHR3.0 into the LS and then performed CSDS (Extended Data Fig. 9a).
We found that inhibition of the NTLS→AHN or NTLS→NAc circuits increased social investigation and partially decreased social avoidance during the RI test (Extended Data Fig. 9b–e).
To test whether these pathways bidirectionally control social preference, we injected either AAV-DIO-ChR2 or AAV-DIO-eNpHR3.0, exposed mice to social defeat stress and then performed optical stimulation of NTLS→AHN or NTLS→NAc circuits during the social conditioning session.
As expected, we found that bidirectional regulation of both pathways affected sCPP (Extended Data Fig. 9f–m).
a, Anterograde AAV-DIO-eYFP tracing from NTLS neurons.b, Anterograde HSV-1 (H129ΔTK-TT) trans-synaptic tracing (70 h post injection) verifies monosynaptic connections between NTLS neurons and regions shown in a.
d–l, ChR2 axon terminal activation in NAc (d), AHN (g) and PAG (j) during RI test in females (NAc (e), social investigation, F (1, 12) = 4.836, P = 0.0482; social avoidance, F (1, 12) = 2.935, P = 0.1123, n = 8 (ChR2), 6 (eYFP); AHN (h), social investigation, F (1, 12) = 4.947, P = 0.0461, social avoidance, F (1, 12) = 0.8571, P = 0.3728, n = 8 (ChR2), 7 (eYFP)); PAG (k), social investigation, F (1, 13) = 0.6986, P = 0.4183; social avoidance, F (1, 13) = 0.07324, P = 0.7909, n = 8 (ChR2), 6 (eYFP); and in males (social investigation, NAc (f), F (1, 13) = 4.540, P = 0.0528; social avoidance, F (1, 13) = 0.2848, P = 0.6026, n = 9 (ChR2), 5 (eYFP); AHN (i), social investigation, F (1, 13) = 28.94, P = 0.0001, social avoidance, F (1, 13) = 0.06521, P = 0.8024, n = 8 (ChR2), 7 (eYFP); PAG (l), social investigation, F (1, 14) = 0.002038, P = 0.9646; social avoidance, F (1, 14) = 1.750, P = 0.2071, n = 9 (ChR2), 6 (eYFP) (f)).
Our data show both pathways to be monosynaptic (with TTX), inhibitory (Cs-based internal, clamped at 0 mV) and GABAa-dependent (SR-95531, Gabazine) (Extended Data Fig. 10a,b).
We also validated that 15 Hz of blue-light stimulation can reliably evoke NTLS neurons (Extended Data Fig. 10c).
Because it has been reported that other cell types in the LS can modulate stress behaviours under different conditions, we tested whether non-NT neurons in the LS also play a role in social trauma-induced social deficits by injecting AAV-Flpo and AAV-CreOff-FlpOn-ChR2 viruses into the LS of Nt-Cre mice to label non-NT neurons with ChR2 (Extended Data Fig. 10d).
We first validated the specificity of this approach using Multiplex ISH (Extended Data Fig. 10e,f), and found very little overlap between ChR2 and NT.
We next validated stimulation parameters for ChR2 using slice electrophysiology and confirmed that 15 Hz reliably activated non-NT neurons in the LS (Extended Data Fig. 10g).
We then stimulated non-NT neurons in the LS in vivo at 15 Hz during the RI test following CSDS and found no effect on social interaction (Extended Data Fig. 10h).
Taken together, these results suggest that the activation of inhibitory NTLS projections to the AHN and NAc is both necessary and sufficient to alter social investigation and social preference of mice following traumatic social experience.
Many components of social behaviour, including its rewarding properties, are evolutionarily conserved between humans and rodents46,47.Although it is well established that social stress leads to the development of depression, anxiety48 and post-traumatic stress disorder38, the neural circuits that mediate the negative consequences of social stress—particularly with regard to social reward—are not well defined.
We view preclinical social stress models as imperative to this early-phase work so that we can define potential circuit mechanisms of trauma-impaired social reward to inform future studies in humans.
Utilizing an unbiased approach, we identified the LS as one of the most highly regulated regions activated in both male and female SUS mice in response to a normally rewarding social target, suggesting that it might be a particularly important region in regard to explaining the common social deficits exhibited by both sexes.In unstressed mice we found these cells to be responsive during situations of threat, including in response to aggressive attack behaviour.
However, following chronic social trauma in SUS mice we found that these neurons generalize their responses to non-threatening social situations, including during interactions with non-aggressive juvenile mice.
Notably, NTLS and Drd3+ neurons exert opposing functions to control social behaviour following stress (Fig. 4 and ref. 26).
Thus, we hypothesized that NTLS neurons might play a unique role in regulation of social reward by inhibiting downstream reward centres.
Indeed, anterograde tracing studies identified known reward centres—including the NAc and AHN—as receiving moderate/dense innervation from NTLS neurons, and activation of these inputs reduced social interaction and context-dependent social reward with a juvenile.
Because anxiety is well known to inhibit adaptive social behaviours; one critical question is whether NTLS neurons are encoding social aversion or whether they simply encode a generalized state of anxiety that impairs social behaviours.According to our data, generalized anxiety states measured by EPM/OFT are separable from social behaviour deficits: (1) when we stimulate NTLS neurons in social stress-naïve mice, we are able to produce a generalized exploratory deficit in the EPM/OFT (Extended Data Fig. 7); however, such stimulation does not induce avoidance of a social target (Extended Data Fig. 6b).
(2) Both RES and SUS mice in the CSDS model exhibited anxiety-like behaviours in the OFT and EPM, yet only SUS mice exhibited social avoidance and reduced social reward.
(3) Although CVS produces an increase in generalized anxiety-like behaviour, it has no effect on social interaction or social reward (Extended Data Fig. 6e,f).
Thus, in addition to regulation of generalized anxiety states, NTLS neurons encode contextual information about stressful/traumatic past experiences to guide future behavioural responses.
Overall our findings demonstrate that, in both male and female SUS mice, rewarding social targets are perceived as stressful or threatening, which engages NTLS circuitry and impairs social reward processing in a context-dependent manner.Our research thus provides an important foundation for understanding the neural mechanisms underlying post-trauma social reward processing.
Future studies in humans to test the relevance of LS circuitry in mediation of social threat perception and reward sensitivity in victims of trauma will be highly informative.
For the juvenile odour-paired CRS, mice were restrained in a 50 mL restrainer and put in a new cage with bedding from a same-sex C57BL/6J juvenile mouse.
We calculated SI ratio as the ratio of time spent in the interaction zone with a CD-1 or F1 ERα-Cre mouse present over time spent with the target absent.
Social investigation included the amount of active interaction including approach, close following and sniffing.
Social avoidance was defined as the escaping from a juvenile mouse of the experimental mouse when approached and investigated by the former.
SUS mice typically escaped at speeds of 20–65 cm s–1 immediately to avoid social encounters following juveniles’ approach/investigation.
The conditioning phase consisted of four consecutive days with two conditioning sessions each day: during the morning paired sessions (08:00–12:00), experimental mice were confined to the assigned chamber for 15 min with a new same-sex juvenile C57BL/6J intruder; during the afternoon unpaired session (13:00–17:00) mice were put into the opposite chamber without a social target for 15 min.
For female sCPP, during conditioning the juvenile mice were confined in a wire-mesh cup, which we found was necessary for females to form CPP, whereas males formed a preference only when they were able to freely interact with the juvenile outside the cup.
Behavioural analysis of sCPP data was performed by assessing (1) subtracted CPP (posttest phase duration spent in the intruder-paired chamber minus test phase duration spent in the intruder-unpaired chamber, accounting for test session behaviour only); and (2) group and individual durations in both pretest and posttest sessions.
Behaviour was tracked using Noldus Ethovision (Noldus Interactive technologies).
Behaviour was tracked using Noldus Ethovision (Noldus Interactive technologies) to record the total distance moved, as well as the time spent in the centre (22 × 22 cm2) versus outer zones.
Male mice were habituated in the testing room for 1 h before testing.
Mice were introduced into the corner of the cage to explore for 30 min with the filter-top covered on the cage.
A 2-day, DREADD-manipulated marble-burying test was performed using a within-subjects design; mice were given either CNO or vehicle in a counterbalanced way, and thus they received CNO or vehicle on the first day and the alternative on the second day.
The RTPP experiments was performed as previously described54: mice were placed in the centre of an arena (44 cm (w) × 44 cm (d) × 35 cm (h)) with a central divider and allowed to explore freely for 20 min.For immunohistochemistry and iDISCO+, mice were euthanized by injection of 10% chloral hydrate and perfused transcardially with ice-cold 1× PBS (pH 7.4), followed by fixation with cold 4% paraformaldehyde in 1× PBS.The z-values in Extended Data Fig.
Nt-Cre mice (4–5 weeks old) were anaesthetized by intraperitoneal injection with a mixture of ketamine HCl (100 mg kg–1) and xylazine (10 mg kg–1) and positioned on a stereotaxic instrument (David Kopf Instruments).For aggressors used in female CSDS, we targeted the VMHvl of ERα-Cre F1 mice as described previously11,58.
For optogenetic (ChR2 and eNpHR3.0) experiments on NTLS terminal stimulation, cannulae (MFC_200/240-0.22_MF1.25_FLT, 5 mm for NAc/AHN, 3 mm for PAG) were implanted into the NAc (from bregma: AP +1.5 mm; ML ±1.5 mm; DV −4.4 mm, 15° angle), the AHN (from bregma: AP −0.7 mm; ML ±1.5 mm; DV −4.8 mm, 10° angle) or PAG (from bregma: AP −4.2 mm; ML ±0.2 mm; DV −2.3 mm).
For ERα-Cre mice (used for female CSDS), CNO (1 mg kg–1, Tocris) was given intraperitoneally 30 min before CSDS11.For all optogenetics tests, experimental mice were habituated to patch cords for 2 days before testing in RI.
For RI experiments, mice were tested over 2 days, counterbalanced under laser-on and -off conditions.
AAV9-hSyn-DIO-EYFP (0.5 ul, 2.0 × 1012 vg mL–1, Addgene) was injected bilaterally into the LS of 4-week-old male Nt-Cre mice.Two to three weeks after injection, the mice underwent CSDS.
Before slice preparation, all mice were exposed to a 4–6-week-old, same-sex juvenile intruder for 5 min.
About 20 min after the RI test, mice were anaesthetized using isoflurane.
For recording of optically evoked inhibitory postsynaptic currents (oIPSCs), AAV9-EF1a-DIO-ChR2-eYFP (0.5 µL, 3.0 × 1012 vg mL–1, Addgene) was injected bilaterally into the LS of 4-week-old male Nt-Cre mice.
NTLS terminals were stimulated through the microscope x40 objective (15 Hz, 5 ms per pulse, 470 nm; no. pE-300ultra, CoolLed).
Three weeks after virus injection and ferrule implantation, when mice were around 8 weeks old, they underwent CSDS and SI; they were then habituated to the patch cord for 2 days and Ca2+ fluorescence was recorded during the RI test, social CPP conditioning session, stress and food reward tests.
Once connected to the apparatus, mice were allowed to rest and habituate for 3–5 min before starting.
For analysis of LS GCaMP6s activity during discrete behaviours in the RI test, average ΔF/F (%) in the 2 s before and after a discrete event (passive social investigation) were compared.
A passive social investigation was determined to occur at the moment of the intruder-initiated passive social approach.
For Extended Data tables and the Supplementary table, P values were corrected for multiple comparisons using the Benjamini–Hochberg procedure to reduce false discovery rate.
Q-values below 0.05 were considered significant for all Extended Data tables.
Figure 2c and Extended Data Fig.Extended Data Fig.
Extended Data Fig.
Extended Data FigsS
Extended Data Fig.
2g,i,j, 3a, 4a and 5a,b and Extended Data Figs.
Peer victimization and dysfunctional reward processing: ERP and behavioral responses to social and monetary rewards.
Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions.
Blunted social reward responsiveness moderates the effect of lifetime social stress exposure on depressive symptoms.
A standardized protocol for repeated social defeat stress in mice.
Establishment of a repeated social defeat stress model in female mice.
Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin.
Gating of social reward by oxytocin in the ventral tegmental area.
Midbrain circuits for defensive behaviourD
Dyadic social interaction as an alternative reward to cocaine.
Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice.
Novel object recognition and object location behavioral testing in mice on a budget.
Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice.
iDISCO+ data were analysed by A.V.A
a—f, SUS mice show higher corner ratio (One-Way ANOVA, female, F (2, 31) = 29.62, P < 0.0001, n = 10 (CTRL), 12 (RES), 12 (SUS) (a), males, F (2, 46) = 17.58, P < 0.0001, n = 10 (CTRL), 13 (RES), 26 (SUS) (d)), while showing no locomotor activity deficits (One-Way ANOVA, female, F (2, 31) = 0.8416, P = 0.4406 (b), males, F (2, 46) = 0.8416, P = 0.4376, (e)) during SI test and showing longer latency to first social bout (One-Way ANOVA, female, F (2, 31) = 8.399, P = 0.0012, (c), males, F (2, 46) = 16.63, P < 0.0001, (f)) during RI testg—l, Correlation between SI ratio and social investigation time (female, R2 = 0.1728, P = 0.0145 (g), male, R2 = 0.1958, P = 0.0015 (j)), social avoidance (female, R2 = 0.3399, P = 0.0003 (h), male, R2 = 0.1847, P = 0.0021 (k)) and latency to first social bout (female, R2 = 0.1464, P = 0.0255 (i), male, R2 = 0.1366, P = 0.0090 (l))
m—r, Correlation between CPP score and social investigation time (female, R2 = 0.2818, P = 0.0012 (m), male, R2 = 0.1533, P = 0.0054 (p)), social avoidance (female, R2 = 0.1995, P = 0.0081 (n), male, R2 = 0.0911, P = 0.0351 (q)) and latency to first social bout (female, R2 = 0.3065, P = 0.0007 (o), male, R2 = 0.0318, P = 0.2200 (r))
Source data
e, Subtracted CPP score (One-Way ANOVA, F (2, 22) = 3.984, P = 0.0334), social investigation time (F (2, 22) = 8.267, P = 0.0021), social avoidance (F (2, 22) = 9.919, P = 0.0008) and SI ratio (F (2, 22) = 18.32, P < 0.0001, n = 8 (CTRL), 7 (RES), 9 (SUS)
f, g, Social behavioural parameters after grouping by CPP score, (f) females, SI ratio (two-tailed t-test, t = 1.660, df = 32, P = 0.1067), social investigation time (t = 3.788, df = 32, P = 0.0006), social avoidance (t = 3.001, df = 32, P = 0.0052), latency (t=3.204, df = 32, P = 0.0031), n = 11 (CPP-), 23 (CPP+)
(g) males, SI ratio (two-tailed t-test, t = 2.298, df = 47, P = 0.0261), social investigation time (t=2.868, df = 47, P = 0.0062), social avoidance (t = 2.545, df = 47, P = 0.0143), latency (t = 1.438, df = 47, P = 0.1570), n = 21 (CPP-), 28 (CPP+)
All data are expressed as mean ± s.e.m
Source data
a—d, Female iDISCO+ groups’ SI ratio (One-Way ANOVA, F (2, 27) = 22.25, P < 0.0001, n = 8 (CTRL), 11 (RES), 11 (SUS)) (a), social investigation time (One-Way ANOVA, F (2, 27) = 20.24, P < 0.0001) (b), social avoidance (One-Way ANOVA, F (2, 27) = 7.747, P = 0.0022) (c) and latency to first social bout (One-Way ANOVA, F (2, 27) = 6.075, P = 0.0066) (d)e—h, Male iDISCO+ groups’ SI ratio (One-Way ANOVA, F (2, 25) = 13.85, P < 0.0001, n = 9 (CTRL), 11 (RES), 8 (SUS)) (e), social investigation time (One-Way ANOVA, F (2, 25) = 14.07, P < 0.0001) (f), social avoidance (One-Way ANOVA, F (2, 25) = 38.76, P < 0.0001) (g) and latency to first social bout (One-Way ANOVA, F (2, 25) = 7.370, P = 0.0030) (h)
All data are expressed as mean ± s.e.m
Source data
a, Ratio of Nt+cFos+ /Nt+ (492 of 520 neurons) and Nt+cFos+ /cFos+ (492 of 496 neurons) in LS of SUS mice(n = 3 slices per mouse, n = 3 mice per group, scale bar, 100 μm)
(n = 3 slices per mouse, n = 3 mice per group, scale bar, 100 μm)
f, g, Multiplex ISH shows Nt and Drd3 do not overlap in the LS (n = 3 slices per mouse, n = 3 mice per group, scale bar, 50 μm)
h, i, Multiplex ISH shows Nt and Oxtr showing very low overlap in the LS (n = 3 slices per mouse, n = 3 mice per group, scale bar, 50 μm)
j, Confocal images of Sst and cFos expression in CTRL, RES and SUS mice after RI test
l, Comparison of Nt− cFos+ neurons between female CTRL, RES and SUS mice (One-Way ANOVA, F (2, 6) = 0.2755, P = 0.7683, n = 3 per group) and males (F (2, 10) = 4.980, P = 0.0316, n = 3–6)
All data are expressed as mean ± s.e.m
Source data
a, Characterization of ex vivo electrophysiology parameters measured in NTLS neurons following CSDS in SUS and RES miceb, Social interaction ratio of fiber photometry cohort after CSDS in females (One-Way ANOVA, F (2, 11) = 5.629, P = 0.0207, n = 4 (CTRL), 5 (RES), 5 (SUS)) and males (One-Way ANOVA, F (2, 14) = 12.93, P = 0.0007, n = 7 (CTRL), 5 (RES), 5 (SUS (5))
c, Ca2+ activity in NTLS neurons during consumption of a peanut butter cup (left)
All data are expressed as mean ± s.e.m
Source data
a, Expression of AAV-DIO-DREADDs in NTLS neuronsb, NTLS activation in stress-naïve mice does not change social behaviour in females (unpaired two-tailed t-test, t14 = 1.044, P = 0.3141, n = 8 per group) or males (unpaired two-tailed t-test, t14 = 1.434, P =0.3975, n = 8 per group)
NTLS activation in stress-naïve mice does not change locomotor activity in females (unpaired two-tailed t-test, t14 = 0.3473, P = 0.7335, n = 8 per group) or males (unpaired two-tailed t-test, t14 = 1.425, P = 0.1762, n = 8 per group)
c, NTLS chemogenetic activation in stress-naïve mice does not change social preference (Two-way repeated measures ANOVA, Vehicle, F = (1, 16) = 7.198, P = 0.0163, n = 9, CNO, F (1, 18) = 6.644, P = 0.0190, n = 10)
g, Schematic diagram of virus injection and optogenetic manipulation of NTLS neurons during RTPP test
h, NTLS neuron activation does not alter real time place preference in either stress-naïve females (Paired two-tailed t-test, ChR2, t11 = 0.06179, P = 0.9518, n = 12, EYFP, t15 = 0.01923, P = 0.9849, n = 16) or males (Paired two-tailed t-test, ChR2, t8 = 0.3855, P = 0.7099, n = 9; EYFP, t8 = 0.6333, P = 0.5442, n = 9)
All data are expressed as mean ± s.e.m
Source data
g, NTLS activation in stress-naïve male mice leads to higher anxiety-like behaviour in the elevated plus maze (unpaired two-tailed t-test, t14 = 2.824, P = 0.0135, n = 8 per group)
h, NTLS activation or inhibition in stress-naïve male mice modulate marble burying behaviour (two-tailed paired t-test, hM3Dq, t7 = 4.631, P = 0.0024, n = 8; hM4Di, t7 = 4.020, P = 0.0051, n = 8)
i, NTLS activation or inhibition in stress-naïve male mice leads to higher or lower anxiety levels, respectively, in the open field test (unpaired two-tailed t-test, hM3Dq, t14 = 2.189, P = 0.0461, n = 8 per group; hM4Di, t14 = 1.424, P = 0.1762, n = 8 per group)
All data are expressed as mean ± s.e.m
Source data
48 h after injection, there were no tdTomato+ neurons in downstream regions of NTLS neurons
c, Colocalization analysis for overlapping NTLS projection neurons to the NAc/AHN/PAG
e, Number of projections showing colocalization of CTB tracers from three downstream regions (3 slices per brain region per mouse, n = 3 mice)
All data are expressed as mean ± s.e.m
a, Schematic of NTLS targeting with NpHR and manipulation during social behaviour testsb–e, NpHR axon terminal inhibition in the NAc (b, c) and AHN (d, e) rescued social investigation time and partially rescued social avoidance in both females (b, social investigation, F (1, 14) = 3.484, P = 0.0831, n = 8 per group; social avoidance, F (1, 14) = 1.180, P = 0.2956, n = 8 per group, d, social investigation, F (1, 14) = 4.982, P = 0.0425, n = 8 per group; social avoidance, F (1, 14) = 2.266, P = 0.1545, n = 8 per group) and males (c, social investigation, F (1, 14) = 0.2046, P = 0.6580, n = 8 per group; social avoidance, F (1, 14) = 1.214, P = 0.2891, n = 8 per group, e, social investigation, F (1, 14) = 4.597, P = 0.0501, n = 8 per group; social avoidance, F (1, 14) = 5.359, P = 0.0363, n = 8 per group)
f, Activation of NTLS→NAc with ChR2 blocked social reward in RES females (EYFP, F (1, 12) = 2.362, P = 0.1502, n = 7, ChR2, F (1, 14) = 0.5543, P = 0.4689, n = 8) g, Inhibition of NTLS→NAc with NpHR rescued social reward deficits in SUS females (EYFP, F (1, 14) = 0.5105, P = 0.4867, NpHR, F (1, 14) = 4.183, P = 0.0601, n = 8 per group)
h, Activation of NTLS→AHN with ChR2 blocked social reward in RES females (EYFP, F (1, 12) = 4.289, P = 0.0606, n = 7, ChR2, F (1, 14) = 0.0001296, P = 0.9911, n = 8)
i, Inhibition of NTLS→AHN with NpHR rescued social reward deficits in SUS females (EYFP, F (1, 14) = 0.1068, P = 0.7487, NpHR, F (1, 14) = 4.575, P = 0.0505, n = 8 per group)
j, Activation of NTLS→NAc with ChR2 blocked social reward in RES males (EYFP, F (1, 12) = 5.703, P = 0.0343, n = 7, ChR2, F (1, 14) = 0.2484, P = 0.6259, n = 8)
k, Inhibition of NTLS→NAc with NpHR rescued social reward deficits in SUS males (EYFP, F (1, 14) = 4.053, P = 0.0637, NpHR, F (1, 14) = 4.140, P = 0.0613, n = 8 per group)
l, Activation of NTLS→AHN with ChR2 blocked social reward in RES males (EYFP, F (1, 12) = 6.329, P = 0.0271, n = 7, ChR2, F (1, 14) = 0.1567, P = 0.6981, n = 8)
m, Inhibition of NTLS→AHN with NpHR rescued social reward deficits in SUS males (EYFP, F (1, 12) = 0.6881, P = 0.4230, NpHR, F (1, 14) = 10.02, P = 0.0069, n = 8 per group)
d, Schematic diagram of non-NTLS neuron infection and manipulation during social behaviour tests
c, Overlap of Nt+ EYFP+ neurons among EYFP+ neurons (2-3 slices per mouse, n = 5 mice)
h, Effect of ChR2 stimulation of non-Nt neurons on social investigation and social avoidance during RI test (Mixed-effects analysis two-way ANOVA, left, F (1, 13) = 0.2137, P = 0.6516, right, F (1, 13) = 0.5672, P = 0.4648, n = ChR2 (10), EYFP (5))
Social trauma engages lateral septum circuitry to occlude social reward