This behaviour is crucial for pupal survival; if the secretion is not removed, pupae develop fungal infections and die.
Analogous to mammalian milk, the secretion is also an important source of early larval nutrition, and young larvae exhibit stunted growth and decreased survival without access to the fluid.
We show that this derived social function of the moulting fluid generalizes across the ants.
Therefore, we cannot fully describe the principles of interactions between individuals and how each developmental stage contributes to and benefits from the social fabric of the colony using observations of intact colonies alone.
biroi pupae outside of the colony in social isolation by providing the abiotic requirements for development (Fig. 1a).
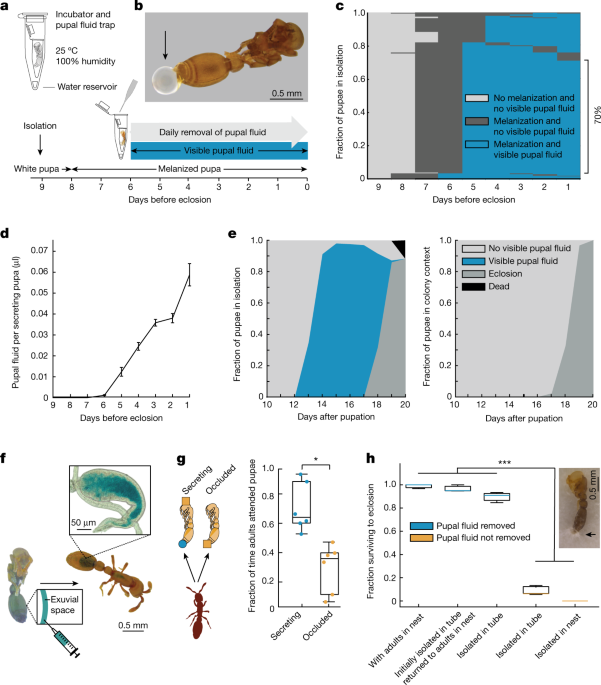
We isolated pupae from the beginning of pupation until eclosion as adults, and found that they secrete a substantial amount of fluid towards the end of metamorphosis (Fig. 1a,b).
In subsequent experiments, we therefore isolated pupae nine days before eclosion and just before melanization.
During this period, on average 99.93 ± 0.22% (mean ± s.d.) of the pupae secrete fluid (n = 9, 142–166 pupae per replicate).
As long as we manually removed fluid daily, our social isolation protocol yielded high survival rates, with 90 ± 3% of pupae eclosing (n = 9, 142–166 pupae per replicate).
Among the pupae that survived to eclosion, we observed a stereotypical pattern: Melanization always preceded secretion, 70% of pupae secreted fluid continuously over the course of five days before eclosion (Fig. 1c), and the average daily volume secreted increased over time, up until eclosion (Fig. 1d).
Throughout the period of secretion, we collected 23.2 ± 1.7 µl of fluid per biological replicate (n = 6, 149–166 pupae per replicate, approximately corresponding to the population of a natural colony of this species).
a, Developmental timeline and protocol for rearing pupae in social isolation (Methods).c,d, Secretion dynamics of isolated pupae over nine days before eclosion.
d, The daily social fluid volume per secreting pupa increases over time (Methods).
e, Dynamics of pupal secretion and eclosion under social isolation (left; n = 942 pupae) and in colonies with adults (right; n = 907 pupae).
g, When given the choice, adults spend more time attending to secreting pupae (blue) compared with pupae prevented from secreting (orange) by occluding the tip of the gaster with a paint dot (orange square) (Methods).
n = 6, with 3 adults, 5 secreting and 5 occluded pupae each.
n = 9 biological replicates for ‘isolated in tube’ with ‘pupal fluid removed’, and n = 3 for all other conditions (30–166 pupae per replicate).
This large amount of fluid secreted by pupae in isolation and the high fraction of pupae secreting daily (Fig. 1e, left) contrasts with the fact that the fluid cannot be readily observed when pupae are kept inside the colony with adult ants (Fig. 1e, right).We therefore tested whether pupae secrete the fluid in the natural colony setting, and if so, what happens to it.
We injected blue food dye into the exuvial space of melanized pupae and traced its distribution in the colony for several days (n = 10 replicate colonies of 10 adults and 10 pupae each) (Fig. 1f).
By the end of the experiment, no pupae had died, and all of them responded to tactile stimulation, showing that adults did not acquire the dye by cannibalizing pupae.
Instead, this suggests that adults consume the pupal fluid immediately as it is secreted, which is why the fluid does not accumulate and become visible in the colony context.
We then isolated pupae and allowed fluid to accumulate before adding adults.
The adults were highly attracted to the fluid and rapidly consumed it (Supplementary Video 2).
Daily manual removal of the secretion was sufficient to achieve high rates of pupal survival and eclosion in isolation, similar to the colony context in which pupae were in a nest with a group of adults (Fig. 1h).
By contrast, if we did not remove fluid from isolated pupae under clean rearing conditions, they drowned in their own secretion (Fig. 1h).
When pupae were isolated in vacant, used nest boxes, the fluid droplets became contaminated with fungi, and these infections spread and ultimately killed all pupae (Fig. 1h).
Pupae that were isolated until fluid droplets appeared and then returned to the nest with a group of adults showed high survival (Fig. 1h).
This shows that, in the colony context, pupae depend on adults to remove the secretion, and would otherwise die of fungal infections.
biroi, pupae produce a type of social fluid that is consumed by the adults.
This fluid is secreted in large volumes by all pupae during a specific window of development, and elicits parental care behaviour that is necessary for pupal survival.
To understand the molecular composition of the pupal social fluid, we collected samples from a population of pupae reared in isolation and conducted proteomic and metabolomic profiling.To verify that the detected compounds were secreted with the fluid, rather than stemming from contamination acquired via contact with the surface of the pupae, we compared the pupal fluid profiles with profiles obtained from pupal whole-body washes.
Hierarchical clustering of proteomic profiles revealed a clear distinction between the pupal fluid and pupal whole-body wash (Fig. 2a and Extended Data Fig. 2).
Out of 212 identified proteins, 185 were either found exclusively or significantly enriched in pupal fluid compared with pupal whole-body wash (t-tests, false discovery rate (FDR) = 0.05) (Supplementary Data 2).
GO terms associated with both pathways are enriched in the pupal fluid (Fig. 2b).
a, Proteomic profiles of pupal social fluid and whole-body wash.b, Proteomic GO enrichment analysis of pupal social fluid (one-sided hypergeometric test, FDR = 0.05).
To provide additional evidence that the pupal fluid is derived from the moulting fluid, we screened for these breakdown products.
Targeted metabolomic profiling of pupal fluid and pupal whole-body wash indicated that the social fluid contains a variety of micro- and macronutrients.
Out of 107 metabolites identified, 105 were significantly enriched in the pupal fluid compared to pupal whole-body wash (t-tests, FDR = 0.05) (Supplementary Data 3).
This is consistent with findings from other insects, where these metabolites increase in the moulting fluid during the moulting process19,24.
These results show that the pupal social fluid is rich in a variety of proteins and metabolites and has the molecular and physiological characteristics of insect moulting fluids.
Non-eusocial Ecdysozoa reabsorb the moulting fluid before ecdysis to recycle nutrients25,26.
By contrast, clonal raider ant pupae secrete a large proportion of the moulting fluid from the pupal case, where it is consumed by adult nestmates.
Given the nutritive content of the pupal social fluid, we hypothesized that larvae, which grow and therefore require the most food, might also consume it.During the brood care phase, colonies contain late instar larvae that induce workers to leave the nest and forage for food.
During the reproductive phase, colonies contain pupae, foraging activity ceases, and workers synchronously lay a new batch of eggs that hatch into larvae at the end of the reproductive phase (Fig. 3a).
Brood therefore develops in discrete cohorts, and each new cohort of larvae hatches while the previous cohort is at the late pupal stage6,27,28.
Thus, the pupal fluid might provide an important food source for larvae before workers begin foraging (approximately the first 4 days of their lives) (Fig. 3a).
To test whether larvae are present during the time in the colony cycle when pupae secrete fluid, we isolated pupae and eggs from colonies in the middle of the reproductive phase and daily recorded the number of pupae secreting and the number of larvae hatching.
The first larvae hatched only one day before pupae began secreting, showing that the two events are tightly synchronized (Fig. 3b).
We then placed larvae in a colony with pupae injected with food dye to test whether the larvae consume dye-stained pupal fluid.
In the colony context, young larvae are usually attached to pupae, often with their mouthparts, suggesting that they consume the liquid directly as it appears on the pupal surface (Supplementary Video 4).
The slice (white outline) represents overlap between larvae and pupae of two subsequent cohorts.
b, Synchronization between larvae hatching (n = 4 replicates with 100 larvae each) and pupae secreting (n = 3 replicates with 162–166 pupae each).
c, Larvae consume pupal social fluid.
d–f, The preference of adults to place larvae on different food sources.
g,h, Larval growth (g) and survival (h) during four days after hatching in colonies with only adults (orange), with adults and prey (grey) or with adults and pupae (blue).
n = 18 colonies with pupae, n = 10 with prey and n = 18 with neither.
i, Contributor-to-beneficiary interactions between pupae, young larvae and adults involving the pupal social fluid.
Pupae secrete their moulting fluid and adults place larvae on pupae.
Larvae and adults consume the fluid, and fluid consumption prevents pupal infections and death.
biroi adults readily place young larvae on pupae and older larvae on prey items (Supplementary Video 5).
To test the adults’ preference for alternative food sources for young larvae, we provided colonies of ten adults and ten newly hatched larvae with either ten melanized O.
biroi pupae, both ten melanized O.
biroi pupae and ten prey items (dead pupae of the fire ant Solenopsis invicta, replaced every other day), or ten prey items alone.
When given pupae alone, ants placed almost all larvae on pupae until eclosion (Fig. 3d).
When given a choice, ants showed a strong preference for placing the larvae on pupae as opposed to prey (Fig. 3e and Extended Data Fig. 6a).
Even in the absence of pupae, ants did not place young larvae on prey (Fig. 3f and Extended Data Fig. 6b).
Thus, ants place newly hatched and early instar larvae on their own pupae, where they have the ability to feed on pupal fluid, and only place later instar larvae on prey (Fig. 3d–f and Extended Data Fig. 6).
To determine whether this relationship contributes to larval growth and survival, we conducted an experiment in which larvae were hatched in social isolation and, at 0–12 h of age and without prior access to food, were fostered into colonies of adult ants.
Colonies were composed of ten adults and five fostered larvae each, and were supplied with either ten melanized O.
biroi pupae, ten prey items (dead S. invicta pupae, replaced every other day), or neither.
Larvae in colonies with pupae grew significantly more (Fig. 3g) and had significantly higher survival (Fig. 3h) compared with larvae in colonies without pupae, regardless of whether prey was present or not.
All pupae in these colonies eclosed, showing that pupae themselves were not consumed (brood cannibalism is a commonly observed response to stressful conditions in ant colonies).
There were no significant differences in growth and survival between larvae in colonies that were given prey and those kept without prey (Fig. 3g,h).
Thus, we observed faster growth and higher survival of larvae reared in the presence of pupae, and found no evidence for a similar beneficial effect of prey items.
These experiments show that the pupal fluid serves as a ‘milk-like’ substance for newly hatched larvae, greatly increasing larval growth and survival during the first days after hatching.
Together, our results reveal that the pupal moulting fluid has a previously unknown yet important role in O.
biroi social organization and colony fitness (Fig. 3i).
Despite its importance in Ooceraea biroi (ant subfamily Dorylinae)—to our knowledge—a social function of pupal secretions as trophallactic fluids has not been described in any of the > 14,000 ant species.biroi, we socially isolated pupae from four other species to cover the five major ant subfamilies: S.
We found that melanized pupae of all four species secrete fluid droplets from the abdominal tip (Fig. 4a).
This shows that, across the ants, pupae secrete a liquid derived from the moulting fluid.
a, Subfamily-level ant phylogeny (top) and pupae of representative species with secretion droplets accumulated over 24 h in social isolation.At least 30 pupae were isolated for each species, with consistent results.
b, Metabolomic profiles of pupal social fluid of the species in a.
invicta adults and early instar larvae take up food dye injected into the exuvial space of pupae.
n = 10 replicates of 10 adults, 10 pupae and 5 early instar larvae each.
d, Survival of pupae to eclosion with (blue) and without (orange) removal of the secretion under the following conditions: with adults in nest (unclean conditions), isolated in tube (isolated in clean conditions), and isolated in nest (isolated in a vacant nest in unclean conditions).
n = 3 biological replicates for ‘isolated in tube’ with ‘pupal fluid not removed’ and n = 4 for all other conditions (30 pupae per replicate).
To test whether pupal fluids also have a social role in other ant species, we injected blue food dye into the exuvial space of melanized S.After 24 h, the digestive systems of all adults and most early instar larvae were stained blue, indicating that they had ingested pupal fluid (Fig. 4c).
invicta pupae do not eclose in social isolation, and concluded that adults are required to help remove the exuvia during eclosion29.
By contrast, while pupae died if their secretion was not removed, manual removal of the social fluid was sufficient to achieve high survival rates without any additional assistance during eclosion (Fig. 4d).
Together, our results show that the secretion of moulting fluid by pupae is widely conserved in ants, and that the dependence of pupae on other colony members to consume the fluid is not limited to O.
In many ant species, adults place young larvae on pupae (Supplementary Video 6), and that adults drink dyed pupal fluid is directly visible in N.
pennsylvanica, adults consume the social fluid through the permeable silken fabric (Extended Data Fig. 8).
Additional work will be required to study the social role of pupal fluids across the ant tree of life.
Although the social functions of the pupal secretion must be a derived trait in ants, it remains to be determined whether pupae of solitary hymenopterans produce secretions derived from the moulting fluid.None of the pupae produced visible secretion droplets (Extended Data Fig. 9), and more than 80% of the pupae survived to eclosion in social isolation without additional assistance.
Although this shows that not all eusocial clades rely on the social functions of pupal fluids, the evolutionary dynamics of pupal secretions across the Hymenoptera will be a rewarding avenue for future investigation.
Here we show that ant pupal moulting fluid has acquired novel social functions that create a previously unrecognized interdependence between pupae, larvae and adults.
We have demonstrated that this fluid is detrimental for pupae if not removed, and that it is an important food source for early larvae.
Similarly, the pupal fluid described here contains hormones and neuroactive substances that may modulate the development and behaviour of larvae and adults (see Supplementary Discussion for details).
biroi colonies, larvae and pupae develop in discrete and synchronized cohorts26.
Ten days after the first larvae had entered pupation in a large stock colony, the entire colony was anaesthetized using a CO2 pad, and white pupae were separated using a paintbrush.
The outer tubes were kept closed throughout the experiment, except for once a day when the tubes were opened to remove pupal social fluid.
To ensure that all secretion had been removed, pupae were taken out of the tube after fluid collection and briefly placed on a tissue paper to absorb any excess liquid.
Control groups of 30 pupae and 30 adult ants from the same stock colony and cohort as the isolated pupae were placed in Petri dishes with a plaster of Paris floor, and the same parameters as for the isolated pupae were scored daily.
A pupa was declared dead if it did not shed its pupal skin and did not respond to touch three days after all pupae in the control group had eclosed.
To calculate the average secretion volume per secreting pupa (Fig. 1d), the total volume collected daily from a group of isolated pupae (142–166 pupae) was divided by the number of pupae from which fluid had been collected that day.While pupae were secreting, pupal whole-body wash samples were collected dailyM
The pupae were removed from colonies with adults and washed promptly with 1500 µl LC–MS grade water.
White pupae were socially isolated, cocoons were removed in the case of P.
mellifera pupae of unknown age were socially isolated from hive fragments (A&Z Apiaries, USA) and reared as described for O biroi, except that the rearing temperature was set to 32 °C.
Pupae were injected with blue food colouring (McCormick) into the exuvium for 1–2 s by gently piercing the pupal case at the abdominal tip with the needle.
During successful injections, no fluid was discharged from the pupa when the needle was removed, and the moulting fluid inside the exuvium was immediately stained.
Following injections, 10 pupae were reared in social isolation to confirm the secretion of dyed droplets.
For experiments, injected pupae were transferred to colonies with adult ants (Figs. 1f and 4c) or to colonies with adult ants and larvae (Figs. 3b and 4c) to track the distribution of the pupal social fluid.
After spending 24 h with dye-injected pupae, adults were taken out of the colony, briefly immersed in 95% ethanol, and transferred to PBS.The area between the pupae was covered with laser-cut filter paper to prevent adults from sticking to the tape.
Pupae were left in isolation for one day before adults were added to the assay chamber.
To assess adult preference (Fig. 1g), physical contact of adults with pupae was manually annotated for the first 10 min after the first adult had encountered (physically contacted) a pupa.
We extracted 30 µl of pupal social fluid and whole-body wash samples with 75:25:0.2 acetonitrile: methanol: formic acid.The list of proteins identified in the pupal fluid was evaluated for functional enrichment in these GO terms, P-values were adjusted with an FDR cut-off of 0.05, and the network plots were visualized using the clusterProfiler package37.
For bulk polar metabolite profiling, we used 10 µl aliquots of pupal social fluid and whole-body wash (pooled samples).For the time-series metabolite profiling, 1 µl of pupal social fluid and whole-body wash was used.
biroi pupae were placed on a rotator to ensure uniform treatment.
biroi pupae.
Bright-field images of pupal fluid droplets secreted from the abdominal tip were acquired with a DMi8 inverted microscope (Leica), an Orca fusion CMOS camera (Hamamatsu) and the VisiTech InstantSIM (iSIM) (VisiTech International) real-time superresolution system with a 20×/0.75 water objective at a resolution of 3.08 pixels per µm.
Figure 1f shows a representative pupa and the crop of a representative adult out of a total of n = 10 replicate colonies of 10 adults and 10 pupae each.
Figure 3c shows a representative larva that has ingested dyed pupal secretion out of n = 3 replicate colonies with 10 larvae, adults and pupae each.The study included one- to several-month-old females, as well as females of different developmental stages (larvae and pupae).
biroi in particular, males are only produced sporadically and do not partake in the social life of the colony.
Trophallaxis: the functions and evolution of social fluid exchange in ant colonies (Hymenoptera: Formicidae)C
Mechanism of chitin hydrolysis by the binary chitinase system in insect moulting fluid.
The disappearance of moulting fluid in the tobacco hornworm, Manduca sexta.
Physiology and biochemistry of insect moulting fluid.
The reproductive cycle of thelytokous colonies of Cerapachys biroi Forel (Formicidae, Cerapachyinae).
biroi pupae.
The rectal invagination is closed in young pupae (a) and only forms a clear opening in older pupae (b).
biroi pupa showing a droplet of pupal fluid secreted from the rectal invagination (orange arrow).
(d) Schematic illustrating the pupal fluid (blue) in the exuvial space surrounding the pupa (top).
The pupal fluid exudes from the rectal invagination of the pupal case (orange arrow, bottom).
biroi show staining of the digestive system only after spending 24 h with pupae that were injected with food dye (right), but not when food dye is offered in a cup for the same amount of time (left).
n = 10 replicate colonies of 10 adults and 10 pupae each.
(a) Colonies of 10 adults and 10 young larvae were given 10 O.biroi pupae and 10 prey items (dead S. invicta pupae).
Adults placed early instar larvae on pupae (left), while prey items were left untouched and intact (right; 2 representative examples from the same colony).
(b) When colonies of 10 Adults and 10 young larvae received 10 prey items only, larvae were often arranged in tight clusters, but not placed on prey.
(c) Adults readily place later instar larvae on prey items (here a dead S. invicta pupa).
The arrowhead indicates the accumulation of pupal fluid.
pennsylvanica cocoon after 1 day in social isolation produces a droplet of pupal fluid on the underside of the slide (arrowhead).
This illustrates that the pupal fluid readily crosses the silken fabric of the cocoon.
(e) A young white pupa (left) and an older melanized pupa (right) of Myrmecocystus mexicanus (subfamily Formicinae) enclosed in cocoons after 1 day in social isolation.
The arrowhead indicates the accumulation of pupal fluid in the melanized pupa, but not the white pupa.
(f) Schematic of dye injection into pupal fluid in species where pupae are enclosed in a cocoon.
mexicanus worker drinking dyed pupal fluid from a cocoon.
(a) Patterns of melanization without visible secretion of individually isolated pupae during development.Pupae of unknown ages were socially isolated and reared until eclosion.
One-sided Student‘s t-test results of proteins in pupal social fluid vs body wash.One-sided Student‘s t-test results of metabolites in pupal social fluid vs body wash.Pairwise comparison results for preference of adults to place larvae on different food sources.Pairwise comparison results for larval growth during 4 days after hatching in a colony with only adults, with adults and prey, and with adults and pupae.Pairwise comparison results for larval survival during 4 days after hatching in a colony with only adults, with adults and prey, and with adults and pupae.invicta pupae to eclosion with and without removal of the pupal fluid.
biroi colony with adults, pupae, and young larvae.
Arrows indicate examples of adults that remain in prolonged physical contact with pupae and are touching pupae with their mouthparts
biroi adults consuming pupal social fluid
biroi pupae were isolated for 1 day to allow fluid to accumulate, and then presented to adults
Preference assay of adults to pupae secreting vsbiroi pupae were occluded with paint on the tip of the gaster (five pupae on the left) or allowed to secrete (five pupae on the right, painted on the head instead)
Pupae were isolated for 1 day and then presented to 3 adults
biroi colony with adults, pupae, and young larvae
Close-up recordings of larvae with their mouthparts attached to pupae
biroi adult placing a young larva on a pupa
biroi pupae were isolated for 1 day, and accumulated fluid was consumed by adults before larvae were added to the experiment
Early instar larvae on pupae in colonies of Nflavipes, older larvae to the right, but not young larvae, are being fed on external food sources stained with blue food dye
flavipes adult consuming dyed pupal social fluid
In this species, the translucent intersegmental membranes of the abdomen in adults allow direct visualization of fluid consumption in the colony context, without prior isolation of pupae
flavipes pupae were injected with blue food dye into their exuvial space and returned to colonies with adults and non-injected pupae
The arrow indicates blue dye visible through the intersegmental membrane after the worker has consumed dyed pupal social fluid
mexicanus adult consuming dyed pupal social fluid through the cocoon
In this species, the translucent cuticle of adults allows direct visualization of fluid consumption
mexicanus pupae were isolated for 1 day to allow accumulation of the secretion outside the pupa but inside the cocoon
We then injected blue food dye through the cocoon and into the pupal secretion before presenting the pupae to adult ants
The arrow indicates blue dye visible through the thorax and inside the ant’s digestive tract after consumption of dyed pupal social fluid through the silken fabric of the cocoon
The pupal moulting fluid has evolved social functions in ants