Here we report the cryo-electron microscopy structure of the wheat CNL Sr355 in complex with the effector AvrSr356 of the wheat stem rust pathogen.
Direct effector binding to the leucine-rich repeats of Sr35 results in the formation of a pentameric Sr35–AvrSr35 complex, which we term the Sr35 resistosome.
Wheat Sr35 and Arabidopsis ZAR1 resistosomes bear striking structural similarities, including an arginine cluster in the leucine-rich repeats domain not previously recognized as conserved, which co-occurs and forms intramolecular interactions with the 'EDVID' motif in the coiled-coil domain.
Electrophysiological measurements show that the Sr35 resistosome exhibits non-selective cation channel activity.
These structural insights allowed us to generate new variants of closely related wheat and barley orphan NLRs that recognize AvrSr35.
Activation of plant NLRs generally leads to an array of immune responses, often linked to rapid host cell death at sites of attempted pathogen infection.
Plant NLRs can be broadly separated into two classes: CNL with an N-terminal coiled-coil domain and TNL with an N-terminal Toll/interleukin 1 receptor (TIR) domain.
tritici (Pgt) threatens global wheat production10, and the emergence of widely virulent Pgt strains, including the Ug99 lineage, has motivated the search for stem rust resistance in wheat germplasm and wild relatives over the past two decades.
The mildew resistance locus A (MLA) receptors of the wheat sister species barley also belong to this group of grass CNLs and share strain-specific immunity with Sr genes18.
Sr35 was first identified in a landrace of the Triticum urartu relative Triticum monococcum (einkorn) and confers immunity to Pgt Ug99 in bread wheat when transferred as a transgene5.
However, because Sr35 was absent in the diploid A genome of the wild ancestor of wheat, T.
urartu, it was initially absent in hexaploid bread wheat (Triticum aestivum).
Sr35 resistance has been linked to the recognition of the Pgt effector AvrSr356, but until now, it has remained inconclusive whether Sr35 receptor-mediated host cell death is driven by direct physical interaction with the AvrSr35 effector6,19.
To purify Sr35, we expressed the protein alone or together with AvrSr35 in Sf21 insect cells.Unexpectedly, cell death was observed when the receptor was co-expressed with AvrSr35 (Extended Data Fig. 1a), suggesting that Sr35 and its effector are sufficient to mediate this immunity-associated response in insect cells in the absence of other plant proteins.
To circumvent cell death activation for protein purification, we introduced substitutions in the N-terminal residues L15E/L19E (Sr35L15E/L19E), which are predicted to be essential for Sr35 membrane association by analogy with the ZAR1 resistosome3.
Mutational analysis of the corresponding N-terminal residues of the tomato CNL NRC4 has been shown to abrogate cell death activity in Nicotiana benthamiana20.
Indeed, the Sr35L15E/L19E substitutions markedly reduced Sr35-induced cell death in insect cells (Extended Data Fig. 1a and Supplementary Table 1).
Using affinity-tagged Sr35L15E/L19E co-expressed with affinity-tagged AvrSr35, we were able to enrich the Sr35–AvrSr35 complex for subsequent separation of potential receptor complex isoforms and correctly folded receptor complexes by size-exclusion chromatography (Extended Data Fig. 1b,c).
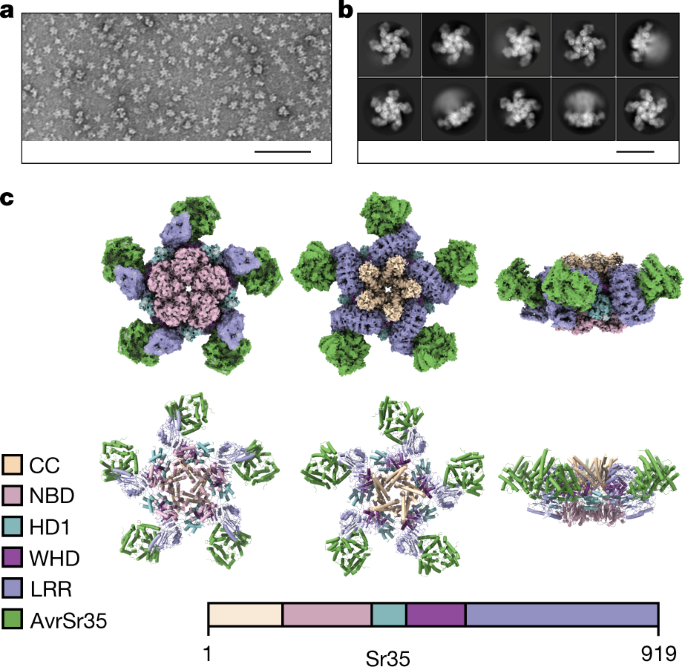
a, Negative staining of purified Sr35 in complex with AvrSr35 (Sr35 resistosome).b, 2D classifications of the Sr35 resistosome particles from the cryo-EM sample.
c, Cryo-EM density map with 3 Å resolution (top) and the finally refined structure model (bottom) of the Sr35 resistosome shown in three different orientations.
AvrSr35 is coloured green and Sr35 domains are shown according to the colour codes in the inset panel.
We analysed the Sr35–AvrSr35 complex sample by cryo-EM (Extended Data Fig. 1) using a total of 1,608,441 individual particles for reference-free two-dimensional (2D) classification (Fig. 1b).Despite the high resolution of up to 2.5 Å in the centre of the complex, the local resolution decreased towards the outer edge to approximately 4 Å (Extended Data Fig. 1f), indicating that the outer region of the complex is more flexible.
To compensate for this decreased resolution, a local mask was used for the outer region, yielding a local density map with a resolution of 3.33 Å (Extended Data Fig. 1f).
The reconstruction revealed a star-shaped structure, similar to the ZAR1 resistosome3, that we termed the Sr35 resistosome.
As in the ZAR1 resistosome, five Sr35 NOD modules define the base of the circular protomer arrangement, and a helical barrel formed by the fivecoiled-coil domains is buried at the centre.
Unlike ZAR1, the leucine-rich repeat (LRR) domains at the outer region do not pack against each other in the Sr35 resistosome, which might explain why this region is more flexible.
AvrSr35 adopts an exclusively helical fold (Extended Data Fig. 2).
The central NOD module of plant NLRs is subdivided into a nucleotide-binding domain (NBD), helical domain 1 (HD1) and winged helical domain (WHD).There is an unambiguous cryo-EM density at the predicted nucleotide-binding site between the HD1 and NBD domains that is unfilled by Sr35 and AvrSr35.
In contrast to that of ZAR1, the ATP in Sr35 does not directly contact the WHD.
a, Sr35 resistosome showing a lateral dimer.Sr35 domains and AvrSr35 coloured according to the inset panel.
b, Structure of one Sr35 protomer in complex with AvrSr35.
f, Structural detail of coiled-coil and LRR domain intramolecular packing in one Sr35 protomer.
g, Cotransfection of Sr35 and Sr35 mutants with AvrSr35 in wheat protoplasts.
Relative luminescence as readout for cell death.
h, Tobacco cell death data of Sr35 and Sr35 mutants with AvrSr35.
i, Wheat protoplast data of EDIVD and R-cluster mutants.
j, Nicotiana benthamiana cell death data of EDVID and R-cluster mutants.
Representative data in h and j shown from seven replicates and scored for leaf cell death.
Similar to the ZAR1 resistosome, NBD–NBD contacts contribute to Sr35 protomer packing (Fig. 2a,d).Of note, the coiled-coil domain of Sr35 contributes considerably to the interprotomer interactions (Fig. 2a): the C-terminal half of α4-helix from one protomer packs against the C-terminal sides of α2- and α4-helices of the neighbouring coiled-coil protomer.
As previously reported22, the long linker region between the coiled-coil and NBD domain is also involved in mediating oligomerization of the Sr35 resistosome.
To functionally test the requirements for these interactions in mediating the assembly of the Sr35 hetero-oligomeric complex, we introduced amino acid substitutions into the receptor and assessed their impact on Sr35-mediated cell death using a luciferase (LUC) activity assay in wheat protoplasts23 prepared from a genotype that does not recognize AvrSr35 (cultivar ‘Chinese Spring’).Cotransfection of Sr35, AvrSr35 and the LUC reporter resulted in a near complete loss of luminescence signal, indicating massive cell death of the protoplasts and suggesting receptor activation by AvrSr35 (Fig. 2g).
Consistent with the insect cell data described above, wheat protoplasts co-expressing Sr35L15E/L19E and AvrSr35 displayed luminescence levels that were comparable to those co-expressing EV and AvrSr35 constructs, indicating that the cell death activity of the Sr35L15E/L19E receptor is substantially impaired (Fig. 2g).
A similarly drastic loss of receptor-mediated cell death activity was observed with substitutions predicted to affect coiled-coil interprotomer interactions (Y141A, L42E and L42E/Y141A) or the ATP-binding site (R311A) (Fig. 2g) (raw data of all protoplast measurements are provided in Supplementary Table 2).
To corroborate the data from wheat protoplasts, we used Agrobacterium tumefaciens-mediated transient gene expression of Sr35 and AvrSr35 in N. benthamiana leaves.Co-expression of Sr35 and AvrSr35, but not AvrSr35 plus EV, resulted in cell death in the Agrobacterium-infiltrated area (Fig. 2h).
By contrast, cell death was abolished when AvrSr35 was co-expressed with the Sr35 mutants predicted to perturb Sr35 oligomerization (Fig. 2h), with the exception of Sr35L42E, which showed residual cell death activity only in N. benthamiana (full versions of all tobacco agroinfiltrations are provided in Supplementary Figs. 1–8).
In planta, protein levels of wild-type Sr35 and all receptor mutants tested were comparable, indicating that these substitutions do not render the receptor unstable (Extended Data Fig. 3a, and full versions of all blots are provided in Supplementary Figs. 9–11).
Together, these data strongly suggest that the residues mediating Sr35 oligomerization in the cryo-EM structure are necessary for cell death activity in wheat and heterologous N. benthamiana.
A conserved sequence in the coiled-coil domain, long known as the ‘EDVID (Glu-Asp-Val-Ile-Asp) motif’ that is present in approximately 38% of Arabidopsis CNLs24 and first described in the potato CNL Rx, is used to group CNLs with or without this motif24,25.In the cryo-EM structure of the Sr35 resistosome, the EDIVD motif (Glu-Asp-Ile-Val-Asp) and the adjacent Sr35 Y74 mediate the packing of the LRR domain against the coiled-coil domain.
Acidic residues from the motif form strong contacts with five arginine residues in the LRR domain (LRRR537, LRRR538, LRRR557, LRRR580 and LRRR602).
These arginine residues are each separated by one iteration of the LRR motif, resulting in their spatial separation along the Sr35 amino acid sequence (Extended Data Fig. 4a).
As previously noted26, the cryo-EM structure of the ZAR1 resistosome shows that similar intramolecular interactions exist between arginine residues in the ZAR1 LRR and ‘EDVID’.
In both resistosomes the respective arginine residues cluster together and form a positively charged surface patch (Extended Data Fig. 4b).
A sequence alignment of CNLs shows that the LRRR-cluster is conserved and co-occurs with the EDVID motif (Extended Data Fig. 4a).
To test whether the LRRR-cluster is necessary for Sr35-mediated cell death, we substituted residues from the interface between the arginine cluster and the EDIVD motif and assessed the impact of these mutations on cell death activity using the wheat protoplast and N.Simultaneous mutations of LRRR537A/R538A in the LRRR-cluster essentially abolished cell death activity (Fig. 2i,j).
Similarly, a triple substitution in the Sr35 EDIVD motif, including the adjacent Sr35 Y74, (Y74A/E77A/D78A) reduced or abolished Sr35 cell death activity in protoplasts and N.
benthamiana, without affecting NLR stability (Extended Data Fig. 3b).
As the EDVID–LRRR-cluster interactions are also present in the inactive ZAR1 and AlphaFold2-modelled Sr35 monomers and an extensive fold switching occurs in the coiled-coil domain during receptor activation (Extended Data Fig. 4c), these intramolecular interactions may be transiently disrupted to allow α1-helix to flip.
Albeit having only 28.4% sequence conservation and although the α1-helix region of Sr35, whose equivalent in ZAR1 resistosome forms a funnel-shaped structure, is not well defined, the structures of the wheat Sr35 and Arabidopsis ZAR1 resistosomes are highly similar (Extended Data Fig. 5).To test this conjecture we used an assay previously established in Xenopus laevis (Xenopus) oocytes4 to assess potential channel activity of the Sr35 resistosome.
Co-expression of Sr35 and AvrSr35, but not either alone, generated currents as recorded by two-electrode voltage-clamp (Fig. 3a,b), suggesting that assembly of the Sr35 resistosome is required for the currents.
In strong support of this conclusion, two Sr35 mutants that impaired the interaction with AvrSr35 and abolished AvrSr35-dependent cell death activity of the receptor in planta (Sr35R730D/R755Q and Sr35W803L/K754G; see below), lost their ability to produce currents in oocytes (Fig. 3c).
In agreement with the data on cell death in planta and insect cells, co-expression of the α1-helix substitution mutant Sr35L15E/L19E with AvrSr35 did not mediate currents in oocytes (Fig. 3c).
Substitutions affecting the acidic inner lining of the funnel formed by α1-helices in ZAR1E11A have been shown to abolish cell death in planta and channel activities in oocytes3,4.
Unexpectedly, both Sr35 resistosome channel and cell death activities were tolerant to these analogous acidic residue substitutions (Sr35E17A/E22A) (Fig. 3c–e and Extended Data Fig. 3c) (raw data of all oocytes measurements are provided in Supplementary Table 4).
a, Representative measurements from two-electrode (TEVC) recordings from Xenopus oocytes expressing Sr35, AvrSr35 and Sr35/AvrSr35.c, Structure-based mutagenesis of Sr35 residues at the interface between the LRR domain of Sr35 and AvrSr35, and Sr35 α1-helix.
d, Wheat protoplast data of Sr35 mutations at α1-helix.
Relative luminescence as readout for cell death.
e, Tobacco cell death data of Sr35 and Sr35 channel mutants.
Together, these results indicate that the Sr35 resistosome may contribute to mixed currents in Xenopus oocytes, possibly via Sr35 resistosome calcium channel activity.
To test whether the Sr35 resistosome can function as a non-selective cation channel, we tested cation flux in the presence of monovalent solutions of potassium and sodium chloride salts (KCl, NaCl).Similar to the ZAR1 resistosome, co-expression of Sr35 and AvrSr35 increased cation flux in oocytes, which was retained for potassium and sodium salts with the immobile gluconate counter-ion (K-Glu, Na-Glu).
A comparison of the divalent ions Ca2+ and Mg2+ (MgCl2, CaCl2) combined with the Sr35 and AvrSr35 co-expression in oocytes, showed that ion flux was significant for calcium but not magnesium (Fig. 3g).
This finding supports the conclusion that the Sr35 resistosome is permeable to calcium.
Although our collective data strongly suggest that the Sr35 resistosome functions similarly to the ZAR1 resistosome by forming a non-selective calcium channel, the channel activity of the Sr35 resistosome is tolerant to substitutions of acidic residues predicted to line the inner surface of the channel.
Thus, we cannot exclude the possibility that the very N terminus of the Sr35 resistosome (residues 1–21) is structurally and functionally distinct from that of the ZAR1 resistosome.
In the cryo-EM structure, AvrSr35 binds to the very C-terminal part of the Sr35 LRR domain, supporting a direct recognition mechanism of AvrSr35 by Sr35 (Fig. 4a).AvrSr35 is much larger than many other pathogen effectors, but only a small portion of the protein is in contact with the Sr35 LRR through charge and shape complementarity (Fig. 4a and Extended Data Fig. 6a,b).
In addition to the hydrophobic and van der Waals interactions, a large network of hydrogen bonds also mediates the Sr35–AvrSr35 interface, supporting specific recognition of AvrSr35 by Sr35 (individual contacts provided in Fig. 4b).
a, Interface between Sr35 LRR and AvrSr35.b, Structural detail of Sr35 receptor and AvrSr35 effector interface.
Grey and white residue label boxes correspond to Sr35 and AvrSr35 sidechains, respectively.
c, Cotransfection of Sr35 LRR mutants with AvrS35 in wheat protoplasts.
Relative luminescence as readout for cell death.
d, Nicotiana benthamiana cell death data of Sr35 LRR mutations at the receptor–effector interface.
Representative data are shown from 11 replicates and scored for leaf cell death.
e, Cotransfection of Sr35 with AvrS35 mutants in wheat protoplasts.
f, Nicotiana benthamiana cell death data of AvrSr35 mutants co-expressed with Sr35.
Representative data are shown from nine replicates and scored for leaf cell death.
To functionally verify the Sr35–AvrSr35 interaction, we first substituted R730, R755 and W803 in Sr35 with their counterparts in the Sr35 homologue of wheat cultivar Chinese Spring27 (here denoted TaSh1), which shares 86.5% sequence identity with Sr35 but is derived from a wheat cultivar susceptible to Pgt strains encoding AvrSr3514.These W803L or R730D substitutions strongly and weakly suppressed Sr35-mediated cell death activity, respectively, when co-expressed with AvrSr35 in wheat protoplasts (Fig. 4c).
By contrast, R755Q had no detectable effect on Sr35-induced cell death, but its combination with R730D resulted in a complete loss of cell death in wheat protoplasts (Fig. 4c).
Similar results were obtained when these Sr35 mutants were assayed in N.
benthamiana (Fig. 4d and Extended Data Fig. 3d).
These data support the Sr35–AvrSr35 interaction in the cryo-EM structure and explain why TaSh1 in susceptible cultivar Chinese Spring is unable to recognize AvrSr35.
To further verify specific AvrSr35 recognition by Sr35, we made the following substitutions in the fungal effector at their interface: Y383A, Y387A, R395A, Y387A/R395A, Y387A/R381A, A384Y/A388Y, all of which either form hydrogen bonds or salt-bridges with the Sr35 LRR (Fig. 4b).
Similar to the Q72* premature stop codon mutant of AvrSr35 (Fig. 4e)6, the mutations Y387A/R395A, Y387A/R381A and A384Y/A388Y abolished Sr35-induced cell death in wheat protoplasts and N. benthamiana (Fig. 4e,f and Extended Data Fig. 3e).
By contrast, single mutations of Y387A and R395A only partially abolished effector-triggered receptor activation (Fig. 4e,f and Extended Data Fig. 3e), and several other single mutations of AvrSr35 (Extended Data Fig. 6b) had no effect, suggesting that much of the AvrSr35–Sr35 interface is resilient to disruption by single amino acid substitutions.
In these predictions, structures of all individual domains were highly similar to those in the Sr35 resistosome and the LRR domain in particular was accurately predicted (Extended Data Fig. 7a).
Although some predictions were a close match with the domain organization of Sr35 in the resistosome, other individual predictions showed striking differences in the domain organization of NOD module (NBD–HD1 relative to WHD) (Extended Data Fig. 7b).
These predictions shared the relative domain organization of inactive, monomeric ZAR1 and other inactive NLR structures29, and most likely represent an inactive Sr35 structure.
Modelling of AvrSr35 onto the LRR domain of the predicted structure of inactive Sr35 shows substantial overlap between the effector and Sr35 NBD (Extended Data Fig. 8).
Comparison of Sr35 and ZAR1 resistosomes suggests that this ‘steric clash’ mechanism is likely to be conserved in CNLs.
AvrSr35 binding dislodges the NBD, allowing subsequent nucleotide exchange for further ATP-triggered allosteric changes in the receptor and assembly of the Sr35 resistosome.
Together, these results support a conserved allosteric mechanism underlying activation of the Sr35 and ZAR1 resistosomes.
Ligand binding to the ascending lateral side of the LRR domain was also seen in the structures of the TNL RPP1 (ref. 30) and Roq1 (ref. 31) resistosomes (Extended Data Fig. 9), suggesting that the ligand binding mechanism may be conserved in plant NLRs29.
To test whether the evolutionary conservation of CNL resistosomes can be harnessed for the design of new receptors with altered function, we first chose two closely related Sr35 homologues (Sh) of unknown resistance function in bread wheat (Triticumaestivum; TaSh1) and in the sister species barley (Hordeumvulgare; HvSh1).We generated hybrid receptors of TaSh1 and HvSh1 in which the LRR domain, including the highly conserved WHD α4-helix, was substituted by the equivalent fragment of Sr35 (termed TaSh1Sr35LRR and HvShSr35LRR) (Fig. 5a and Extended Data Fig. 10a).
Unlike wild-type TaSh1 or HvSh1 genes, both hybrid receptors mediated AvrSr35-dependent cell death in wheat leaf protoplasts prepared from cultivar Chinese Spring and when expressed in leaves of N. benthamiana (Fig. 5b–d), indicating neofunctionalization of the orphan receptors.
a, Illustration of Sr35 domain structure and hybrid receptors made from Sr35 homologues (Sh) in bread wheat (Triticum aestivum; TaSh1) and barley (Hordeum vulgare; HvSh1).b, Wheat protoplast transfections of TaSh1Sr35LRR, HvSh1Sr35LRR and controls co-expressed with AvrSr35.
c, Tobacco cell death of TaSh1Sr35LRR and HvSh1Sr35LRR.
Representative data shown from seven replicates and scored for cell death.
e, Cryo-EM structure of Sr35 and structural predictions of TaSH1LRR and HvSH1LRR (ref. 28).
f, Wheat protoplast transfections of TaSh1GOF, HvSh1GOF and controls co-expressed with AvrSr35.
g, Tobacco cell death of TaSh1GOF and HvSh1GOF.
j, Wheat protoplast transfections of HvMla10Sr35LRR, HvMla13Sr35LRR and controls co-expressed with AvrSr35.
k, Tobacco cell death of HvMla10Sr35LRR and HvMla13Sr35LRR.
monococcum Sr35 (86.5% and 86.4% amino acid sequence identity to Sr35, respectively), we reasoned that targeted amino acid substitutions in the LRR domains of the homologues might be sufficient to enable detection of AvrSr35.
Combined structural model (Extended Data Fig. 10b,c) and protein sequence alignments indicated that the AvrSr35-interacting residues of Sr35 are polymorphic in TaSH1 and HvSH1 (Fig. 5e and Extended Data Fig. 10d).
Unlike wild-type HvSh1, HvSh1GOF mediated a clear cell death response in wheat protoplasts and N. benthamiana when co-expressed with the effector AvrSr35 (Fig. 5f–h).
TaSh1GOF induced a notable cell death phenotype in wheat protoplasts, but not in N. benthamiana (Fig. 5f–h), which is probably due to undetectable TaSH1GOF protein in the heterologous N. benthamiana expression system (Fig. 5h).
The relatively small number of nucleotide changes needed to enable TaSH1 to detect AvrSr35 makes it feasible to introduce such changes in elite bread wheat by gene editing.
Next, we investigated whether the Sr35 LRR domain, transferred to more distant CNLs (approximately 45% amino acid sequence identity) in the sister species barley, can generate functional hybrid receptors.The two resulting hybrid receptors, HvMla10Sr35LRR and HvMla13Sr35LRR, induced cell death when co-expressed with Pgt AvrSr35 in wheat protoplasts and N. benthamiana (Fig. 5j–l).
Three independent lines of evidence support this idea: (1) our structural elucidation of the wheat Sr35 resistosome and its similarity to the previously reported Arabidopsis ZAR1 resistosome structure3; (2) the functional interspecies hybrid receptors generated from the non-orthologous CNLs wheat Sr35 and barley MLAs; and (3) the conservation extends to the non-selective cation flux across membranes enabled by pentamerization, although it is possible that ion selectivity and channel dynamics differ between individual CNLs, including the channel activity of so-called helper NLRs acting downstream of canonical plant NLRs39.
Reconstitution of effector-dependent Sr35-triggered cell death in insect cells indicates that regulated channel activity is sufficient to recapitulate plant CNL-mediated cell death in eukaryotic cells of another kingdom.
It is possible that plant CNL pore formation and ion flux trigger and intersect with intrinsic cell death pathways in animals, for example, Apaf-1 apoptosome-mediated developmental and stress-induced cell death40,41.
Although the components needed for cell death downstream of CNL channel activity in plants remain to be identified, the evolutionary conservation of channel activity rationalizes how highly diverse pathogen signals activate a shared set of downstream responses.
Our data support a similar mechanism for Sr35 activation by direct recognition of AvrSr35.
Although AvrSr35 is essential for the activation of Sr35, the effector makes no contribution to oligomerization of the Sr35 resistosome, which is principally mediated by the conserved NBD.
This is also true for the assembly of the ZAR1 resistosome in Arabidopsis and the Apaf-1 apoptosome in animals3,42.
Our findings allow the prediction not only of AvrSr35 substitutions that might escape Sr35 recognition, but also substitutions in the Sr35 LRR that can physically ‘re-capture’ such effector variants.
More generally, the evolutionarily conserved plant CNL resistosome architecture with its conserved function highlights the future potential of structure-guided NLR engineering for crop improvement. Note added in proof: After completion of this work, the Sr35 resistosome structure was confirmed in an independent study49.
There were five Sr35 molecules in the complex, each of which was bound to one AvrSr35 molecule.
Model statistics can be found in Extended Data Table 1.
Seedlings of the wheat cultivar.Quantities of receptor-encoding pIPKb002 plasmid were varied for each construct in an effort to minimize cell death due to (receptor) toxicity-mediated cell death (EV 8 µg; Sr35 and Sr35 mutants 2 µg; AvrSr35 and AvrSr35 mutants 5 ug; HvMla10, HvMla13, HvMla10Sr35LRR, HvMla13Sr35LRR, TaSh1, TaSh1, TaSh1GOF, TaSh1GOF 8 µg; TaSh1Sr35LRR and TaSh1Sr35LRR 2 µg).
A maximum of two technical replicates were completed with the same batch of wheat seedlings.
For N. benthamiana transient gene expression, Sr35 and Sr35 mutants, AvrSr35 and AvrSr35 mutants were cloned into the pDONR vector (Invitrogen).The obtained plasmids of Sr35 and Sr35 mutants were recombined by an LR clonase II (Thermo Fisher Scientific) reaction into pGWB517-4×Myc with a C-terminally fused 4×Myc epitope tag64, while AvrSr35 and AvrSr35 mutants were recombined into the pXCSG-mYFP65 vector with a C-terminally fused mYFP epitope tag.
For Sr35 and Sr35 substitution mutants, the OD600 was adjusted to 0.15.
In the hybrid receptor gain-of-function experiment, the OD600 of TaSh1, HvSh1, TaSh1GOF and HvSh1GOF bacterial strains was adjusted to 1.8 without resulting in cell death in co-expression of TaSh1 and HvSh1 when co-expressed with AvrSr35.
For phenotypic experiments, Agrobacteria cultures expressing receptor constructs, or the respective receptor mutants, were co-infiltrated with AvrSr35, or its mutants, at 1:1 ratio using a syringe.
The cDNAs of Sr35, or Sr35 mutants, and AvrSr35 were cloned into the pGHME2 plasmid for expression in Xenopus oocytes.
Both the amount of cRNA injected and the oocyte incubation time were optimized to minimize toxicity caused by the assembled Sr35 resistosome.
Isolated oocytes were co-injected with 0.5 ng cRNA of Sr35 (WT and mutants) and AvrSr35.
Data were analysed using OriginPro, 2022 (OriginLab).
I–V relations for Sr35 resistosome channels were generated from currents that were measured 0.2 s by the end of each test voltage step.
Purification of the Sr35 resistosome was performed more than 10 times.Pull-down and SDS analysis were highly reproducible between biological replicates and comparable with Extended Data Fig.
Cryo-EM datasets were recorded twice from independent protein preparations (micrograph of one cryo-EM sample preparation shown in Extended Data Fig. 1d) and yielded highly similar cryo-EM density maps.
Insect cell death data were performed with six biological replicates and yielded comparable results to Extended Data Fig.Tobacco agroinfiltration data was performed with at least two biological replicates for each substitution mutant and always simultaneously with western blot analysis.Technical replicates of one dataset are shown as raw image data.
Western blot samples were always obtained from the same biological replicate as the phenotypic data.
Only phenotypic data for which the western blot gave a clear signal are shown.
The atomic coordinates of the Sr35 resistosome have been deposited in the Protein Data Bank (PDB) with the accession code 7XC2GThe EM map for the local mask of Sr35 LRR in complex with AvrSr35 has been deposited in the Electron Microscopy Data Bank (EMDB) with the accession code EMD-33111.
Source data of tobacco agroinfiltrations, western blots, insect cell viability and wheat protoplast cell death are provided with this manuscriptL
Reconstitution and structure of a plant NLR resistosome conferring immunity.
The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling.
Identification of wheat gene Sr35 that confers resistance to Ug99 stem rust race groupP
Variation in the AvrSr35 gene determines Sr35 resistance against wheat stem rust race Ug99.
Differential EDS1 requirement for cell death activities of plant TIR-domain proteins.
The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production.
Identification and characterization of Sr13, a tetraploid wheat gene that confers resistance to the Ug99 stem rust race group.
The gene Sr33, an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99.
The wheat Sr50 gene reveals rich diversity at a cereal disease resistance locus.
Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement.
A recombined Sr26 and Sr61 disease resistance gene stack in wheat encodes unrelated NLR genes.
Dissection of cell death induction by wheat stem rust resistance protein Sr35 and its matching effector AvrSr35.
A cell death assay in barley and wheat protoplasts for identification and validation of matching pathogen AVR effector and plant NLR immune receptors.
A semi-dominant NLR allele causes whole-seedling necrosis in wheat.
Highly accurate protein structure prediction with AlphaFold.
Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ.
The dual role of calcium as messenger and stressor in cell damage, death, and survival.
Atomic structure of the apoptosome: mechanism of cytochrome c-and dATP-mediated activation of Apaf-1.
Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism.
RELION: implementation of a Bayesian approach to cryo-EM structure determination.
A Bayesian view on cryo-EM structure determination.
PHENIX: a comprehensive Python-based system for macromolecular structure solution.
We acknowledge Zhengzhou University and EMBL Heidelberg for their assistance with cryo-EM data acquisitionacquired the structure
assisted during structure study
analysed the data
a, Cell viability data in Sf21 insect cellsf, Flowchart of cryo-EM data processing and Sr35-AvrSr35 3D reconstruction
a, Sr35 NBD ATP-binding and coiled-coil protomer interface mutants
b, Sr35 EDVID and arginine-cluster mutants
c, Sr35 channel mutants
d, Sr35 LRR mutants
e, AvrSr35 mutants
a, Multiple protein sequence alignment of HvMLA10, HvMLA13, Sr35 and ZAR1c, Structural alignment of Sr35 inactive structure prediction (cyan) and one protomer (yellow) from Sr35 resistosome
In analogy to ZAR1, the Sr35 coiled-coil (CC) α1-helix might undergo structural rearrangement, which likely requires EDVID with arginine cluster interactions to transiently resolve
The structures (in surface representation) of the ZAR1 resistosome and the Sr35 resistosome are shownSr35 is directly activated by the fungal effector AvrSr35
a, Shape and charge complementarity of Sr35 LRR and AvrSr35 at their interface. (Left) AvSr35 shown as cartoon (lime) and Sr35 as electrostatics surface model(Right) Sr35 LRR shown as cartoon (cyan) and AvrSr35 as electrostatics surface model
b, Wheat protoplast data of AvrSr35 mutants predicted to impair Sr35 recognition
Relative luminescence as readout for cell death
a, Structural alignment of WHD and LRR domains from Sr35 AlphaFold2 prediction (cyan) and from Sr35 resistosome Cryo-EM structure (blue)b, Structural comparison of monomeric Sr35 from prediction (left) and from Cryo-EM structure (right)
Inactive Sr35 inside the cell comes in contact with Pgt effector AvrSr35. In avoidance of a steric clash (red) between AvrSr35 and the Sr35 NBD domain, the Sr35 NBD domain is forced to structurally rearrange and a ‘primed’ receptor-effector complex is formedFull activation and oligomerization requires subsequent ADP release, ATP binding and, NOD module rearrangement and coiled-coil (CC) domain structural rearrangement
Sr35 domains and AvrSr35 are coloured according to in-figure legend
Ligand binding to LRR of CNLs (Zar1, Sr35) and LRR-CJID of TNLs (Roq1, RPP1) occurs in equivalent region in the ascending lateral side of the LRR domain (compare concave, convex, ascending and descending lateral sides defined on Zar1)Structure of Sr35 is isolated from the cryo-EM Sr35 resistosome structure, while HvMLA10, HvMLA13, TaSH1 and HvSH1 were predicted using AlphaFold2
Raw data of insect cell viability corresponding to Extended Data Figure 1aRaw data for all protoplast measurements presented in the main figuresRaw data of the electrophysiology data shown in FigAlphaFold2 structure predictions of Sr35, TaSH1, HvSH1, HvMLA10 and HvMLA13A wheat resistosome defines common principles of immune receptor channels